Corey Is Trying to Cut Back on His Consumption of Caffeinated Beverages. He Will Not Experience:
Start Preamble Start Printed Page 53688
AGENCY:
Drug Enforcement Administration, Department of Justice.
ACTION:
Denial of petition to initiate proceedings to reschedule marijuana.
SUMMARY:
By letter dated July 19, 2016 the Drug Enforcement Administration (DEA) denied a petition to initiate rulemaking proceedings to reschedule marijuana. Because the DEA believes that this matter is of particular interest to members of the public, the agency is publishing below the letter sent to the petitioner which denied the petition, along with the supporting documentation that was attached to the letter.
DATES:
August 12, 2016.
Start Further Info
FOR FURTHER INFORMATION CONTACT:
Michael J. Lewis, Office of Diversion Control, Drug Enforcement Administration; Mailing Address: 8701 Morrissette Drive, Springfield, Virginia 22152; Telephone: (202) 598-6812.
End Further Info End Preamble
SUPPLEMENTARY INFORMATION:
July 19, 2016
Dear Ms. Raimondo and Mr. Inslee:
On November 30, 2011, your predecessors, The Honorable Lincoln D. Chafee and The Honorable Christine O. Gregoire, petitioned the Drug Enforcement Administration (DEA) to initiate rulemaking proceedings under the rescheduling provisions of the Controlled Substances Act (CSA). Specifically, your predecessors petitioned the DEA to have marijuana and "related items" removed from Schedule I of the CSA and rescheduled as medical cannabis in Schedule II.
Your predecessors requested that the DEA remove marijuana and related items from Schedule I based on their assertion that:
(1) Cannabis has accepted medical use in the United States;
(2) Cannabis is safe for use under medical supervision;
(3) Cannabis for medical purposes has a relatively low potential for abuse, especially in comparison with other Schedule II drugs.
In accordance with the CSA rescheduling provisions, after gathering the necessary data, the DEA requested a scientific and medical evaluation and scheduling recommendation from the Department of Health and Human Services (HHS). The HHS concluded that marijuana has a high potential for abuse, has no accepted medical use in the United States, and lacks an acceptable level of safety for use even under medical supervision. Therefore, the HHS recommended that marijuana remain in Schedule I. The scientific and medical evaluation and scheduling recommendation that the HHS submitted to the DEA is enclosed with this letter.
Based on the HHS evaluation and all other relevant data, the DEA has concluded that there is no substantial evidence that marijuana should be removed from Schedule I. A document prepared by the DEA addressing these materials in detail also is enclosed. In short, marijuana continues to meet the criteria for Schedule I control under the CSA because:
(1) Marijuana has a high potential for abuse. The HHS evaluation and the additional data gathered by the DEA show that marijuana has a high potential for abuse.
(2) Marijuana has no currently accepted medical use in treatment in the United States. Based on the established five-part test for making such determination, marijuana has no "currently accepted medical use" because: As detailed in the HHS evaluation, the drug's chemistry is not known and reproducible; there are no adequate safety studies; there are no adequate and well-controlled studies proving efficacy; the drug is not accepted by qualified experts; and the scientific evidence is not widely available.
(3) Marijuana lacks accepted safety for use under medical supervision. At present, there are no marijuana products approved by the U.S. Food and Drug Administration (FDA), nor is marijuana under a New Drug Application (NDA) evaluation at the FDA for any indication. The HHS evaluation states that marijuana does not have a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions. At this time, the known risks of marijuana use have not been shown to be outweighed by specific benefits in well-controlled clinical trials that scientifically evaluate safety and efficacy.
The statutory mandate of Title 21 United States Code, Section 812(b) (21 U.S.C. 812(b)) is dispositive. Congress established only one schedule, Schedule I, for drugs of abuse with "no currently accepted medical use in treatment in the United States" and "lack of accepted safety for use . . . under medical supervision." 21 U.S.C. 812(b).
Although the HHS evaluation and all other relevant data lead to the conclusion that marijuana must remain in schedule I, it should also be noted that, in view of United States obligations under international drug control treaties, marijuana cannot be placed in a schedule less restrictive than schedule II. This is explained in detail in accompanying document titled "Preliminary Note Regarding Treaty Considerations."
Accordingly, and as set forth in detail in the accompanying HHS and DEA documents, there is no statutory basis under the CSA for the DEA to grant your predecessors' petition to initiate rulemaking proceedings to reschedule marijuana. The petition is, therefore, hereby denied.
Sincerely,
Chuck Rosenberg,
Acting Administrator.
Attachments:
Preliminary Note Regarding Treaty Considerations
Cover Letter from HHS to DEA Summarizing the Scientific and Medical Evaluation and Scheduling Recommendation for Marijuana.
U.S. Department of Health and Human Services (HHS)—Basis for the Recommendation for Maintaining Marijuana in Schedule I of the Controlled Substances Act
U.S. Department of Justice—Drug Enforcement Administration (DEA), Schedule of Controlled Substances: Maintaining Marijuana in Schedule I of the Controlled Substances Act, Background, Data, and Analysis: Eight Factors Determinative of Control and Findings Pursuant to 21 U.S.C. 812(b)
Start Signature
Dated: July 19, 2016.
Chuck Rosenberg,
Acting Administrator, Preliminary Note Regarding Treaty Considerations.
End Signature
As the Controlled Substances Act (CSA) recognizes, the United States is a party to the Single Convention on Narcotic Drugs, 1961 (referred to here as the Single Convention or the treaty). 21 U.S.C. 801(7). Parties to the Single Convention are obligated to maintain various control provisions related to the drugs that are covered by the treaty. Many of the provisions of the CSA were enacted by Congress for the specific purpose of ensuring U.S. compliance with the treaty. Among these is a scheduling provision, 21 U.S.C. 811(d)(1). Section 811(d)(1) provides that, where a drug is subject to control under the Single Convention, the DEA Administrator (by delegation from the Attorney General) must "issue an order controlling such drug under the schedule he deems most appropriate to carry out such [treaty] obligations, without regard to the findings required by [21 U.S.C. 811(a) or 812(b)] and without regard to the procedures prescribed by [21 U.S.C. 811(a) and (b)]."
Marijuana is a drug listed in the Single Convention. The Single Convention uses the term "cannabis" to refer to marijuana.[1] Thus, the DEA Start Printed Page 53689 Administrator is obligated under section 811(d) to control marijuana in the schedule that he deems most appropriate to carry out the U.S. obligations under the Single Convention. It has been established in prior marijuana rescheduling proceedings that placement of marijuana in either schedule I or schedule II of the CSA is "necessary as well as sufficient to satisfy our international obligations" under the Single Convention. NORML v. DEA, 559 F.2d 735, 751 (D.C. Cir. 1977). As the United States Court of Appeals for the DC Circuit has stated, "several requirements imposed by the Single Convention would not be met if cannabis and cannabis resin were placed in CSA schedule III, IV, or V."[2] Id. Therefore, in accordance with section 811(d)(1), DEA must place marijuana in either schedule I or schedule II.
Because schedules I and II are the only possible schedules in which marijuana may be placed, for purposes of evaluating this scheduling petition, it is essential to understand the differences between the criteria for placement of a substance in schedule I and those for placement in schedule II. These criteria are set forth in 21 U.S.C. 812(b)(1) and (b)(2), respectively. As indicated therein, substances in both schedule I and schedule II share the characteristic of "a high potential for abuse." Where the distinction lies is that schedule I drugs have "no currently accepted medical use in treatment in the United States" and "a lack of accepted safety for use of the drug . . . under medical supervision," while schedule II drugs do have "a currently accepted medical use in treatment in the United States."[3]
Accordingly, in view of section 811(d)(1), this scheduling petition turns on whether marijuana has a currently accepted medical use in treatment in the United States. If it does not, DEA must, pursuant to section 811(d), deny the petition and keep marijuana in schedule I.
As indicated, where section 811(d)(1) applies to a drug that is the subject of a rescheduling petition, the DEA Administrator must issue an order controlling the drug under the schedule he deems most appropriate to carry out United States obligations under the Single Convention, without regard to the findings required by sections 811(a) or 812(b) and without regard to the procedures prescribed by sections 811(a) and (b). Thus, since the only determinative issue in evaluating the present scheduling petition is whether marijuana has a currently accepted medical use in treatment in the United States, DEA need not consider the findings of sections 811(a) or 812(b) that have no bearing on that determination, and DEA likewise need not follow the procedures prescribed by sections 811(a) and (b) with respect to such irrelevant findings. Specifically, DEA need not evaluate the relative abuse potential of marijuana or the relative extent to which abuse of marijuana may lead to physical or psychological dependence.
As explained below, the medical and scientific evaluation and scheduling recommendation issued by the Secretary of Health and Human Services concludes that marijuana has no currently accepted medical use in treatment in the United States, and the DEA Administrator likewise so concludes. For the reasons just indicated, no further analysis beyond this consideration is required. Nonetheless, because of the widespread public interest in understanding all the facts relating to the harms associated with marijuana, DEA is publishing here the entire medical and scientific analysis and scheduling evaluation issued by the Secretary, as well as DEA's additional analysis.
Department of Health and Human Services, Office of the Secretary Assistant Secretary for Health, Office of Public Health and Science, Washington, DC 20201.
June 25, 2015.
The Honorable Chuck Rosenberg
Acting Administrator, Drug Enforcement Administration, U.S. Department of Justice, 8701 Morrissette Drive, Springfield, VA 22152.
Dear Mr. Rosenberg:
Pursuant to the Controlled Substances Act (CSA, 21 U.S.C. § 811(b), (c), and (f)), the Department of Health and Human Services (HHS) is recommending that marijuana continue to be maintained in Schedule I of the CSA.
The Food and Drug Administration (FDA) has considered the abuse potential and dependence-producing characteristics of marijuana.
Marijuana meets the three criteria for placing a substance in Schedule I of the CSA under 21 U.S.C. 812(b)(1). As discussed in the enclosed analyses, marijuana has a high potential for abuse, no currently accepted medical use in treatment in the United States, and a lack of accepted safety for use under medical supervision. Accordingly, HHS recommends that marijuana be maintained in Schedule I of the CSA. Enclosed are two documents prepared by FDA's Controlled Substance Staff (in response to petitions filed in 2009 by Mr. Bryan Krumm and in 2011 by Governors Lincoln D. Chafee and Christine O. Gregoire) that form the basis for the recommendation. Pursuant to the requests in the petitions, FDA broadly evaluated marijuana, and did not focus its evaluation on particular strains of marijuana or components or derivatives of marijuana.
FDA's Center for Drug Evaluation and Research's current review of the available evidence and the published clinical studies on marijuana demonstrated that since our 2006 scientific and medical evaluation and scheduling recommendation responding to a previous DEA petition, research with marijuana has progressed. However, the available evidence is not sufficient to determine that marijuana has an accepted medical use. Therefore, more research is needed into marijuana's effects, including potential medical uses for marijuana and its derivatives. Based on the current review, we identified several methodological challenges in the marijuana studies published in the literature. We recommend they be addressed in future clinical studies with marijuana to ensure that valid scientific data are generated in studies evaluating marijuana's safety and efficacy for therapeutic use. For example, we recommend that studies need to focus on consistent administration and reproducible dosing of marijuana, potentially through the use of administration methods other than smoking. A summary of our review of the published literature on the clinical uses of marijuana, including recommendations for future studies, is attached to this document.
FDA and the National Institutes of Health's National Institute on Drug Abuse (NIDA) also believe that work continues to be needed to ensure support by the federal government for the efficient conduct of clinical research using marijuana. Concerns have been raised about whether the existing federal regulatory system is flexible enough to respond to increased interest in research into the potential therapeutic uses of marijuana and marijuana-derived drugs. HHS welcomes an opportunity to continue to explore these concerns with DEA.
Should you have any questions regarding theses recommendations, please contact Corinne P. Moody, Science Policy Analyst, Controlled Substances Staff, Center for Drug Evaluation and Research, FDA, at (301) 796-3152.
Sincerely yours,
Karen B. DeSalvo, MD, MPH, MSc
Acting Assistant Secretary for Health.
Enclosure: Basis for the Recommendation for Maintaining Marijuana in Schedule I of the Controlled Substances Act
Start Printed Page 53690
Basis for the Recommendation for Maintaining Marijuana in Schedule I of the Controlled Substances Act
On November 30, 2011, Governors Lincoln D. Chafee of Rhode Island and Christine O. Gregoire of Washington submitted a petition to the Drug Enforcement Administration (DEA) requesting that proceeding be initiated to repeal the rules and regulations that place marijuana[4] in Schedule I of the Controlled Substances Act (CSA). The petition contends that cannabis has an accepted medical use in the United States, is safe for use under medical supervision, and has a relatively low abuse potential compared to other Schedule II drugs. The petition requests that marijuana and "related items" be rescheduled in Schedule II of the CSA. In June 2013, the DEA Administrator requested that the U.S. Department of Health and Human Services (HHS) provide a scientific and medical evaluation of the available information and a scheduling recommendation for marijuana, in accordance with the provisions of 21 U.S.C. 811(b).
In accordance with 21 U.S.C. 811(b), DEA has gathered information related to the control of marijuana (Cannabis sativa)[5] under the CSA. Pursuant to 21 U.S.C. 811(b), the Secretary of HHS is required to consider in a scientific and medical evaluation eight factors determinative of control under the CSA. Following consideration of the eight factors, if it is appropriate, the Secretary must make three findings to recommend scheduling a substance in the CSA. The findings relate to a substance's abuse potential, legitimate medical use, and safety or dependence liability.
Administrative responsibilities for evaluating a substance for control under the CSA are performed by the Food and Drug Administration (FDA), with the concurrence of the National Institute on Drug Abuse (NIDA), as described in the Memorandum of Understanding (MOU) of March 8, 1985 (50 FR 9518-20).
In this document, FDA recommends the continued control of marijuana in Schedule I of the CSA. Pursuant to 21 U.S.C. 811(c), the eight factors pertaining to the scheduling of marijuana are considered below.
1. Its Actual or Relative Potential for Abuse
Under the first factor the Secretary must consider marijuana's actual or relative potential for abuse. The CSA does not define the term "abuse." However, the CSA's legislative history suggests the following in determining whether a particular drug or substance has a potential for abuse:[6]
a. There is evidence that individuals are taking the drug or drugs containing such a substance in amounts sufficient to create a hazard to their health or to the safety of other individuals or to the community.
b. There is a significant diversion of the drug or drugs containing such a substance from legitimate drug channels.
c. Individuals are taking the drug or drugs containing such a substance on their own initiative rather than on the basis of medical advice from a practitioner licensed by law to administer such drugs in the course of his professional practice.
d. The drug or drugs containing such a substance are new drugs so related in their action to a drug or drugs already listed as having a potential for abuse to make it likely that the drug will have the same potentiality for abuse as such drugs, thus making it reasonable to assume that there may be significant diversions from legitimate channels, significant use contrary to or without medical advice, or that it has a substantial capability of creating hazards to the health of the user or to the safety of the community.
In the development of this scientific and medical evaluation for the purpose of scheduling, the Secretary analyzed considerable data related to the substance's abuse potential. The data include a discussion of the prevalence and frequency of use, the amount of the substance available for illicit use, the ease of obtaining or manufacturing the substance, the reputation or status of the substance "on the street," and evidence relevant to at-risk populations. Importantly, the petitioners define marijuana as including all Cannabis cultivated strains. Different marijuana samples derived from various cultivated strains may have very different chemical constituents, thus the analysis is based on what is known about the range of these constituents across all cultivated strains.
Determining the abuse potential of a substance is complex with many dimensions, and no single test or assessment provides a complete characterization. Thus, no single measure of abuse potential is ideal. Scientifically, a comprehensive evaluation of the relative abuse potential of a substance can include consideration of the following elements: Receptor binding affinity, preclinical pharmacology, reinforcing effects, discriminative stimulus effects, dependence producing potential, pharmacokinetics, route of administration, toxicity, data on actual abuse, clinical abuse potential studies, and public health risks. Importantly, abuse can exist independently from tolerance or physical dependence because individuals may abuse drugs in doses or patterns that do not induce these phenomena. Additionally evidence of clandestine population and illicit trafficking of a substance can shed light on both the demand for a substance as well as the ease of obtaining a substance. Animal and human laboratory data and epidemiological data are all used in determining a substance's abuse potential. Moreover, epidemiological data can indicate actual abuse.
The petitioners compare the effects of marijuana to currently controlled Schedule II substances and make repeated claims about their comparative effects. Comparisons between marijuana and the diverse array of Schedule II substances is difficult, because of the pharmacologically dissimilar actions of substances of Schedule II of the CSA. For example, Schedule II substances include stimulant-like drugs (e.g., cocaine, methylphenidate, and amphetamine), opioids (e.g., oxycodone, fentanyl), sedatives (e.g., pentobarbital, amobarbital), dissociative anesthetics (e.g., PCP), and naturally occurring plant components (e.g., coca leaves and poppy straw). The mechanism(s) of action of the above Schedule II substances are wholly different from one another, and they are different from tetrahydrocannabinol (THC) and marijuana as well. For example, Schedule II stimulants typically function by increasing monoaminergic tone via an increase in dopamine and norepinephrine (Schmitt et al., 2013). In contrast, opioid analgesics function via mu-opioid receptor agonist effects. These differing mechanism(s) of action result in vastly different behavioral and adverse effect profiles, making comparisons across the range of Start Printed Page 53691 pharmacologically diverse C-II substances inappropriate.
In addition, many substances scheduled under the CSA are reviewed and evaluated within the context of commercial drug development, using data submitted in the form of a new drug application (NDA). A new analgesic drug might be compared to a currently scheduled analgesic drug as part of the assessment of its relative abuse potential. However, because the petitioners have not identified a specific indication for the use of marijuana, identifying an appropriate comparator based on indication cannot be done.
a. There is evidence that individuals are taking the substance in amounts sufficient to create a hazard to their health or to the safety of other individuals or to the community.
Evidence shows that some individuals are taking marijuana in amounts sufficient to create a hazard to their health and to the safety of other individuals and the community. A large number of individuals use marijuana. HHS provides data on the extent of marijuana abuse through NIDA and the Substance Abuse and Mental Health Services Administration (SAMHSA). According to the most recent data from SAMHSA's 2012 National Survey on Drug Use and Health (NSDUH), which estimates the number of individuals who have used a substance within a month prior to the study (described as "current use"), marijuana is the most commonly used illicit drug among Americans aged 12 years and older, with an estimated 18.9 million Americans having used marijuana within the month prior to the 2012 NSDUH. Compared to 2004, when an estimated 14.6 million individuals reported using marijuana within the month prior to the study, the estimated rates in 2012 show an increase of approximately 4.3 million individuals. The 2013 Monitoring the Future (MTF) survey of 8th, 10th, and 12th grade students also indicates that marijuana is the most widely used illicit substance in this age group. Specifically, current month use was at 7.0 percent of 8th graders, 18.0 percent of 10th, graders and 22.7 percent of 12th graders. Additionally, the 2011 Treatment Episode Data Set (TEDS) reported that primary marijuana abuse accounted for 18.1 percent of non-private substance-abuse treatment facility admissions, with 24.3 percent of those admitted reporting daily use. However, of these admissions for primary marijuana abuse, the criminal justice system referred 51.6 percent to treatment. SAMHSA's Drug Abuse Warning Network (DAWN) was a national probability survey of U.S. hospitals with emergency departments (EDs) and was designed to obtain information on ED visits in which marijuana was mentioned, accounting for 36.4 percent of illicit drug related ED visits. There are some limitations related to DAWN data on ED visits, which are discussed in detail in Factor 4, "Its History and Current Pattern of Abuse;" Factor 5, "The Scope, Duration, and Significance of Abuse;" and Factor 6, "What, if any, Risk There is to the Public Health." These factors contain detailed discussions of these data.
A number of risks can occur with both acute and chronic use of marijuana. Detailed discussions of the risks are addressed in Factor 2, "Scientific Evidence of its Pharmacological Effect, if Known," and Factor 6, "What, if any, Risk There is to the Public Health."
b. There is significant diversion of the substance from legitimate drug channels.
There is a lack of evidence of significant diversion of marijuana from legitimate drug channels, but this is likely due to the fact that marijuana is more widely available from illicit sources rather than through legitimate channels. Marijuana is not an FDA-approved drug product, as an NDA or biologics license application (BLA) has not been approved for marketing in the United States. Numerous states and the District of Columbia have state-level medical marijuana laws that allow for marijuana use within that state. These state-level drug channels do not have sufficient collection of data related to medical treatment, including efficacy and safety.
Marijuana is used by researchers for nonclinical research as well as clinical research under investigational new drug (IND) applications; this represents the only legitimate drug channel in the United States. However, marijuana used for research represents a very small contribution of the total amount of marijuana available in the United States, and thus provides limited information about diversion. In addition, the lack of significant diversion of investigation supplies is likely because of the widespread availability of illicit marijuana of equal or greater amounts of delta9-THC. The data originating from the DEA on seizure statistics demonstrate the magnitude of the availability for illicit marijuana. DEA's System to Retrieve Information from Drug Evidence (STRIDE) provides information on total domestic drug seizures. STRIDE reports a total domestic seizure of 573,195 kg of marijuana in 2011, the most recent year with complete data that is currently publically available (DEA Domestic Drug Seizures, n.d.).
c. Individuals are taking the substance on their own initiative rather than on the basis of medical advice from a practitioner licensed by law to administer such substances.
Because the FDA has not approved an NDA or BLA for a marijuana drug product for any therapeutic indication, the only way an individual can take marijuana on the basis of medical advice through legitimate channels at the federal level is by participating in research under an IND application. That said, numerous states and the District of Columbia have passed state-level medical marijuana laws allowing for individuals to use marijuana under certain circumstances. However, data are not yet available to determine the number of individuals using marijuana under these state-level medical marijuana laws. Regardless, according to the 2012 NSDUH data, 18.9 million American adults currently use marijuana (SAMHSA, 2013). Based on the large number of individuals reporting current use of marijuana and the lack of an FDA-approved drug product in the United States, one can assume that it is likely that the majority of individuals using marijuana do so on their own initiative rather than on the basis of medical advice from a licensed practitioner.
d. The substance is so related in its action to a substance already listed as having a potential for abuse to make it likely that it will have the same potential for abuse as such substance, thus making it reasonable to assume that there may be significant diversions from legitimate channels, significant use contrary to or without medical advice, or that it has a substantial capability of creating hazards to the health of the user or to the safety of the community.
FDA has approved two drug products containing cannabinoid compounds that are structurally related to the active components in marijuana. These two marketed products are controlled under the CSA. Once a specific drug product containing cannabinoids becomes approved, that specific drug product may be moved from Schedule I to a different Schedule (II-V) under the CSA. Firstly, Marinol—generically known as dronabinol—is a Schedule III drug product containing synthetic delta9-THC. Marinol, which is formulated in sesame oil in soft gelatin capsules, was first placed in Schedule II under the CSA following its approval by the FDA. Marinol was later rescheduled to Schedule III under the CSA because of low numbers of reports of abuse relative to marijuana. Dronabinol is Start Printed Page 53692 listed in Schedule I under the CSA. FDA approved Marinol in 1985 for the treatment of nausea and vomiting associated with cancer chemotherapy in patients who failed to respond adequately to conventional anti-emetic treatments. In 1992, FDA approved Marional for anorexia associated with weight loss in patients with acquired immunodeficiency syndrome (AIDS). Secondly, in 1985, FDA approved Cesamet, a drug product containing the Schedule II substance nabilone, for the treatment of nausea and vomiting associated with cancer chemotherapy. Besides the two cannabinoid-containing drug products FDA approved for marketing, other naturally occurring cannabinoids and their derivatives (from Cannabis) and their synthetic equivalents with similar chemical structure and pharmacological activity are included in the CSA as Schedule I substances.
2. Scientific Evidence of Its Pharmacological Effects, if Known
Under the second factor, the Secretary must consider the scientific evidence of marijuana's pharmacological effects. Abundant scientific data are available on the neurochemistry, toxicology, and pharmacology of marijuana. This section includes a scientific evaluation of marijuana's neurochemistry; pharmacology; and human and animal behavioral, central nervous system, cognitive, cardiovascular, autonomic, endocrinological, and immunological system effects. The overview presented below relies upon the most current research literature on cannabinoids.
Neurochemistry and Pharmacology of Marijuana
Marijuana is a plant that contains numerous natural constituents, such as cannabinoids, that have a variety of pharmacological actions. The petition defines marijuana as including all Cannabis cultivated strains. Different marijuana samples derived from various cultivated strains may have very different chemical constituents including delta9-THC and other cannabinoids (Appendino et al., 2011). As a consequence, marijuana products from different strains will have different biological and pharmacological profiles.
According to ElSohly and Slade (2005) and Appendino et al. (2011), marijuana contains approximately 525 identified natural constituents, including approximately 100 compounds classified as cannabinoids. Cannabinoids primarily exist in Cannabis, and published data suggests that most major cannabinoid compounds occurring naturally have been identified chemically. New and minor cannabinoids and other new compounds are continuously being characterized (Pollastro et al., 2011). So far, only two cannabinoids (cannabigerol and its corresponding acid) have been obtained from a non-Cannabis source. A South African Helichrysum (H. umbraculigerum) accumulates these compounds (Appendino et al., 2011). The chemistry of marijuana is described in more detail in Factor 3, "The State of Current Scientific Knowledge Regarding the Drug or Other Substance."
The site of cannabinoid action is at the cannabinoid receptors. Cloning of cannabinoid receptors, first from rat brain tissue (Matsuda et al., 1990) and then from human brain tissue (Gerard et al., 1991), has verified the site of action. Two cannabinoid receptors, CB1 and CB2, were characterized (Battista et al., 2012; Piomelli, 2005). Evidence of a third cannabinoid receptor exists, but it has not been identified (Battista et al., 2012).
The cannabinoid receptors, CB1 and CB2, belong to the family of G-protein-coupled receptors, and present a typical seven transmembrane-spanning domain structure. Cannabinoid receptors link to an inhibitory G-protein (Gi), such that adenylate cyclase activity is inhibited when a ligand binds to the receptor. This, in turn, prevents the conversion of ATP to the second messenger, cyclic AMP (cAMP). Examples of inhibitory coupled receptors include opioid, muscarinic cholinergic, alpha2-adrenoreceptors, dopamine (D2), and serotonin (5-HT1).
Cannabinoid receptor activation inhibits N- and P/Q-type calcium channels and activates inwardly rectifying potassium channels (Mackie et al., 1995; Twitchell et al., 1997). N-type calcium channel inhibition decreases neurotransmitter release from several tissues. Thus, calcium channel inhibition may be the mechanism by which cannabinoids inhibit acetylcholine, norepinephrine, and glutamate release from specific areas of the brain. These effects may represent a potential cellular mechanism underlying cannabinoids' antinociceptive and psychoactive effects (Ameri, 1999).
CB1 receptors are found primarily in the central nervous system, but are also present in peripheral tissues. CB1 receptors are located mainly in the basal ganglia, hippocampus, and cerebellum of the brain (Howlett et al., 2004). The localization of these receptors may explain cannabinoid interference with movement coordination and effects on memory and cognition. Additionally, CB1 receptors are found in the immune system and numerous other peripheral tissues (Petrocellis and Di Marzo, 2009). However, the concentration of CB1 receptors is considerably lower in peripheral tissues than in the central nervous system (Herkenharn et al., 1990 and 1992).
CB2 receptors are found primarily in the immune system, but are also present in the central nervous system and other peripheral tissues. In the immune system, CB2 receptors are found predominantly in B lymphocytes and natural killer cells (Bouaboula et al., 1993). CB2 receptors may mediate cannabinoids' immunological effects (Galiegue et al., 1995). Additionally, CB2 receptors have been localized in the brain, primarily in the cerebellum and hippocampus (Gong et al., 2006). The distribution of CB2 receptors throughout the body is less extensive than the distribution of CB1 receptors (Petrocellis and Di Marzo, 2009). However, both CB1 and CB2 receptors are present in numerous tissues of the body.
Cannabinoid receptors have endogenous ligands. In 1992 and 1995, two endogenous cannabinoid receptor agonists, anandamide and arachidonyl glycerol (2-AG), respectively, were identified (Di Marzo, 2006). Anandamide is a low efficacy agonist (Breivogel and Childers, 2000) and 2-AG is a high efficacy agonist (Gonsiorek et al., 2000). Cannabinoid endogenous ligands are present in central as well as peripheral tissues. A combination of uptake and hydrolysis terminate the action of the endogenous ligands. The endogenous cannabinoid system is a locally active signaling system that, to help restore homeostasis, is activated "on demand" in response to changes to the local homeostasis (Petrocellis and Di Marzo, 2009). The endogenous cannabinoid system, including the endogenous cannabinoids and the cannabinoid receptors, demonstrate substantial plasticity in response to several physiological and pathological stimuli (Petrocellis and Di Marzo, 2009). This plasticity is particularly evident in the central nervous system.
Delta9-THC and cannabidiol (CBD) are two abundant cannabinoids present in marijuana. Marijuana's major psychoactive cannabinoid is delta9-THC (Wachtel et al., 2002). In 1964, Gaoni and Mechoularn first described delta9-THC's structure and function. In 1963, Mechoularn and Shvo first described CBD's structure. The pharmacological actions of CBD have not been fully studied in humans.
Delta9-THC and CBD have varying affinity and effects at the cannabinoid receptors. Delta9-THC displays similar Start Printed Page 53693 affinity for CB1 and CB2 receptors, but behaves as a weak agonist for CB2 receptors. The identification of synthetic cannabinoid ligands that selectively bind to CB2 receptors but do not have the typical delta9-THC-like psychoactive properties suggests that the activation of CB1-receptors mediates cannabinoids' psychotropic effects (Hanus et al., 1999). CBD has low affinity for both CB1 and CB2 receptors (Mechoulam et al., 2007). According to Mechoulam et al. (2007), CBD has antagonistic effects at CB1 receptors and some inverse agonistic properties at CB2 receptors. When cannabinoids are given subacutely to rats, CB1 receptors down-regulate and the binding of the second messenger system coupled to CB1 receptors, GTPgarnmaS, decreases (Breivogel et al., 2001).
Animal Behavioral Effects
Self-Administration
Self-administration is a method that assesses the ability of a drug to produce rewarding effects. The presence of rewarding effects increases the likelihood of behavioral responses to obtain additional drug. Animal self-administration of a drug is often useful in predicting rewarding effects in humans, and is indicative of abuse liability. A good correlation is often observed between those drugs that rhesus monkeys self-administer and those drugs that humans abuse (Balster and Bigelow, 2003). Initially, researchers could not establish self-administration of cannabinoids, including delta9-THC, in animal models. However, self-administration of delta9 -THC can now be established in a variety of animal models under specific training paradigms (Justinova et al., 2003, 2004, 2005).
Squirrel monkeys, with and without prior exposure to other drugs of abuse, self-administer delta9-THC under specific conditions. For instance, Tanda et al. (2000) observed that when squirrel monkeys are initially trained to self-administer intravenous cocaine, they will continue to bar-press delta9-THC at the same rate as they would with cocaine. The doses were notably comparable to those doses used by humans who smoke marijuana. SR141716, a CB1 cannabinoid receptor agonist-antagonist, can block this rewarding effect. Other studies show that naïve squirrel monkeys can be successfully trained to self-administer delta9-THC intravenously (Justinova et al., 2003). The maximal responding rate is 4 μg/kg per injection, which is 2-3 times greater than observed in previous studies using cocaine-experienced monkeys. Naltrexone, a mu-opioid antagonist, partially antagonizes these rewarding effects of delta9-THC (Justinova et al., 2004).
Additionally, data demonstrate that under specific conditions, rodents self-administer cannabinoids. Rats will self-administer delta9-THC when applied intracerebroventricularly (i.c.v.), but only at the lowest doses tested (0.01-0.02 μg/infusion) (Braida et al., 2004). SR141716 and the opioid antagonist naloxone can antagonize this effect. However, most studies involve rodents self-administrating the synthetic cannabinoid WIN 55212, a CB1 receptor agonist with a non-cannabinoid structure (Deiana et al., 2007; Fattore et al., 2007; Martellotta et al., 1998; Mendizabal et al., 2006).
Aversive effects, rather than reinforcing effects, occur in rats that received high doses of WIN 55212 (Chaperon et al., 1998) or delta9-THC (Sanudo-Pena et al., 1997), indicating a possible critical dose-dependent effect. In both studies, SR141716 reversed these aversive effects.
Conditioned Place Preference
Conditioned place preference (CPP) is a less rigorous method than self-administration for determining whether or not a drug has rewarding properties. In this behavioral test, animals spend time in two distinct environments: one where they previously received a drug and one where they received a placebo. If the drug is reinforcing, animals will choose to spend more time in the environment paired with the drug, rather than with the placebo, when presented with both options s.imultaneously.
Animals show CPP to delta9-THC, but only at the lowest doses tested (0.075-1.0 mg/kg, intraperitoneal (i.p.)) (Braida et al., 2004). SR141716 and naloxone antagonize this effect (Braida et al., 2004). As a partial agonist, SR141716 can induce CPP at doses of 0.25, 0.5, 2 and 3 mg/kg (Cheer et al., 2000). In knockout mice, those without μ-opioid receptors do not develop CPP to delta9-THC (Ghozland et al., 2002).
Drug Discrimination Studies
Drug discrimination is a method where animals indicate whether a test drug produces physical or psychic perceptions similar to those produced by a known drug of abuse. In this test, an animal learns to press one bar when it receives the known drug of abuse and another bar when it receives placebo. To determine whether the test drug is like the known drug of abuse, a challenge session with the test drug demonstrates which of the two bars the animal presses more often.
In addition to humans (Lile et al., 2009; Lile et al., 2011), it has been noted that animals, including monkeys (McMahon, 2009), mice (McMahon et al., 2008), and rats (Gold et al., 1992), are able to discriminate cannabinoids from other drugs or placebo. Moreover, the major active metabolite of delta9-THC, 11-hydroxy-delta9-THC, also generalizes (following oral administration) to the stimulus cues elicited by delta9-THC (Browne and Weissman, 1981). Twenty-two other cannabinoids found in marijuana also fully substitute for delta9-THC. However, CBD does not substitute for delta9-THC in rats (Vann et al., 2008).
Discriminative stimulus effects of delta9-THC are pharmacologically specific for marijuana containing cannabinoids (Balster and Prescott, 1992; Browne and Weissman, 1981; Wiley et al., 1993, 1995). The discriminative stimulus effects of the cannabinoid group appear to provide unique effects because stimulants, hallucinogens, opioids, benzodiazepines, barbiturates, NMDA antagonists, and antipsychotics do not fully substitute for delta9-THC.
Central Nervous System Effects
Human Physiological and Psychological Effects
Psychoactive Effects
Below is a list of the common subjective responses to cannabinoids (Adams and Martin, 1996; Gonzalez, 2007; Hollister 1986, 1988; Institute of Medicine, 1982). According to Maldonado (2002), these responses to marijuana are pleasurable to many humans and are often associated with drug-seeking and drug-taking. High levels of positive psychoactive effects are associated with increased marijuana use, abuse, and dependence (Scherrer et al., 2009; Zeiger et al., 2010).
(1) Disinhibition, relaxation, increased sociability, and talkativeness.
(2) Increased merriment and appetite, and even exhilaration at high doses.
(3) Enhanced sensory perception, which can generate an increased appreciation of music, art, and touch.
(4) Heightened imagination, which can lead to a subjective sense of increased creativity.
(5) Initial dizziness, nausea, tachycardia, facial flushing, dry mouth, and tremor.
(6) Disorganized thinking, inability to converse logically, time distortions, and short-term memory impairment.
(7) Ataxia and impaired judgment, which can impede driving ability or Start Printed Page 53694 lead to an increase in risk-tasking behavior.
(8) Illusions, delusions, and hallucinations that intensify with higher doses.
(9) Emotional lability, incongruity of affect, dysphoria, agitation, paranoia, confusion, drowsiness, and panic attacks, which are more common in inexperienced or high-dosed users.
As with many psychoactive drugs, a person's medical, psychiatric, and drug-taking history can influence the individual's response to marijuana. Dose preferences to marijuana occur in that marijuana users prefer higher concentrations of the principal psychoactive substance (1.95 percent delta9-THC) over lower concentrations (0.63 percent delta9-THC) (Chait and Burke, 1994). Nonetheless, frequent marijuana users (>100 times of use) were able to identify a drug effect from low-dose delta9-THC better than occasional users (<10 times of use) while also experiencing fewer sedative effects from marijuana (Kirk and de Wit, 1999).
The petitioners contend that many of marijuana's naturally occurring cannabinoids mitigate the psychoactive effects of delta9-THC, and therefore that marijuana lacks sufficient abuse potential to warrant Schedule I placement, because Marinol, which is in Schedule III, contains only delta9-THC. This theory has not been demonstrated in controlled studies. Moreover, the concept of abuse potential encompasses all properties of a substance, including its chemistry, pharmacology, and pharmacokinetics, as well as usage patterns and diversion history. The abuse potential of a substance is associated with the repeated or sporadic use of a substance in nonmedical situations for the psychoactive effects the substance produces. These psychoactive effects include euphoria, perceptual and other cognitive distortions, hallucinations, and mood changes. However, as stated above, the abuse potential not only includes the psychoactive effects, but also includes other aspects related to a substance.
DEA's final published rule entitled "Rescheduling of the Food and Drug Administration Approved Product Containing Synthetic Dronabinol [(-)-delta9-(trans)-Tetrahydrocannabinol] in Sesame Oil and Encapsulated in Soft Gelatin Capsules From Schedule II to Schedule III" (64 FR 35928, July 2, 1999) rescheduled Marinol from Schedule II to Schedule III. The HHS assessment of the abuse potential and subsequent scheduling recommendation compared Marinol to marijuana on different aspects related to abuse potential. Major differences in formulation, availability, and usage between marijuana and the drug product, Marinol, contribute to their differing abuse potentials.
Hollister and Gillespie (1973) estimated that delta9-THC by smoking is 2.6 to 3 times more potent than delta9-THC ingested orally. The intense psychoactive drug effect achieved, rapidly by smoking is generally considered to produce the effect desired by the abuser. This effect explains why abusers often prefer to administer certain drugs by inhalation, intravenously, or intranasally rather than orally. Such is the case with cocaine, opium, heroin, phencyclidine, methamphetamine, and delta9-THC from marijuana (0.1-9.5 percent delta9-THC range) or hashish (10-30 percent delta9-THC range) (Wesson and Washburn, 1990). Thus, the delayed onset and longer duration of action for Marinol may be contributing factors limiting the abuse or appeal of Marinol as a drug of abuse relative to marijuana.
The formulation of Marinol is a factor that contributes to differential scheduling of Marinol and marijuana. For example, extraction and purification of dronabinol from the encapsulated sesame oil mixture of Marinol is highly complex and difficult. Additionally, the presence of sesame oil mixture in the formulation may preclude the smoking of Marinol-laced cigarettes.
Additionally, there is a dramatic difference between actual abuse and illicit trafficking of Marinol and marijuana. Despite Marinol's availability in the United States, there have been no significant reports of abuse, diversion, or public health problems due to Marinol. By comparison, 18.9 million American adults report currently using marijuana (SAMHSA, 2013).
In addition, FDA's approval of an NDA for Marinol allowed for Marinol to be rescheduled to Schedule II, and subsequently to Schedule III of the CSA. In conclusion, marijuana and Marinol differ on a wide variety of factors that contribute to each substance's abuse potential. These differences are major reasons distinguishing the higher abuse potential for marijuana and the different scheduling determinations of marijuana and Marinol.
In terms of the petitioners' claim that different cannabinoids present in marijuana mitigate the psychoactive effects of delta9-THC, only three of the cannabinoids present in marijuana were simultaneously administered with delta9-THC to examine how the combinations of these cannabinoids such as CBD, cannabichromene (CBC) and cannabinol (CBN) influence delta9-THC's psychoactive effects. Dalton et al. (1976) observed that smoked administration of placebo marijuana cigarettes containing injections of 0.15 mg/kg CBD combined with 0.025mg/kg of delta9-THC, in a 7:1 ratio of CBD to delta9-THC, significantly decreased ratings of acute subjective effects and "high" when compared to smoking delta9-THC alone. In contrast, Ilan et al. (2005) calculated the naturally occurring concentrations of CBC and CBD in a batch of marijuana cigarettes with either 1.8 percent or 3.6 percent delta9-THC concentration by weight. For each strength of delta9-THC in marijuana cigarettes, the concentrations of CBC and CBD were classified in groups of either low or high. The study varied the amount of CBC and CBD within each strength of delta9-THC marijuana cigarettes, with administrations consisting of either low CBC (between 0.1-0.2 percent CBC concentration by weight) and low CBD (between 0.1-0.4 percent CBD concentration by weight), high CBC (>0.5 percent CBC concentration by weight) and low CBD, or low CBC and high CBD (>1.0 percent CBD concentration by weight). Overall, all combinations scored significantly greater than placebo on ratings of subjective effects, and there was no significant difference between any combinations.
The oral administration of a combination of either 15, 30, or 60 mg CBD with 30 mg delta9-THC dissolved in liquid (in a ratio of at least 1:2 CBD to delta9-THC) reduced the subjective effects produced by delta9-THC alone (Karniol et al., 1974). Additionally, orally administering a liquid mixture combining 1 mg/kg CBD with 0.5 mg/kg of delta9-THC (ratio of 2:1 CBD to delta9-THC) decreased scores of anxiety and marijuana drug effect on the Addiction Research Center Inventory (ARCI) compared to delta9-THC alone (Zuardi et al.,1982). Lastly, oral administration of either 12.5, 25, or 50 mg CBN combined with 25 mg delta9-THC dissolved in liquid (ratio of at least 1:2 CBN to delta9-THC) significantly increased subjective ratings of "drugged," "drowsy," "dizzy," and "drunk," compared to delta9-THC alone (Karniol et al., 1975).
Even though some studies suggest that CBD may decrease some of delta9-THC's psychoactive effects, the ratios of CBD to delta9-THC administered in these studies are not present in marijuana used by most people. For example, in one study, researchers used smoked marijuana with ratios of CBD to delta9-THC naturally present in marijuana Start Printed Page 53695 plant material and they found out that varying the amount of CBD actually had no effect on delta9-THC's psychoactive effects (Ilan et al., 2005). Because most marijuana currently available on the street has high amounts of delta9-THC with low amounts of CBD and other cannabinoids, most individuals use marijuana with low levels of CBD present (Mehmedic et al., 2010). Thus, any possible mitigation of delta9-THC's psychoactive effects by CBD will not occur for most marijuana users. In contrast, one study indicated that another cannabinoid present in marijuana, CBN, may enhance delta9-THC's psychoactive effects (Karniol et al., 1975).
Behavioral Impairment
Marijuana induces various psychoactive effects that can lead to behavioral impairment. Marijuana's acute effects can significantly interfere with a person's ability to learn in the classroom or to operate motor vehicles. Acute administration of smoked marijuana impairs performance on learning, associative processes, and psychomotor behavioral tests (Block et al., 1992). Ramaekers et al. (2006a) showed that acute administration of 250 μg/kg and 500 μg/kg of delta9-THC in smoked marijuana dose-dependently impairs cognition and motor control, including motor impulsivity and tracking impairments (Ramaekers et al., 2006b). Similarly, administration of 290 μg/kg delta9-THC in a smoked marijuana cigarette resulted in impaired perceptual motor speed and accuracy: Two skills which are critical to driving ability (Kurzthaler et al., 1999). Lastly, administration of 3.95 percent delta9-THC in a smoked marijuana cigarette not only increased disequilibrium measures, but also increased the latency in a task of simulated vehicle braking at a rate comparable to an increase in stopping distance of five feet at 60 mph (Liguori et al., 1998). However, acute administration of marijuana containing 2.1 percent delta9-THC does not produce "hangover effects" (Chait, 1990).
In addition to measuring the acute effects immediately following marijuana administration, researchers have conducted studies to determine how long behavioral impairments last after abstinence. Some of marijuana's acute effects may not fully resolve until at least one day after the acute psychoactive effects have subsided. Heishman et al. (1990) showed that impairment on memory tasks persists for 24 hours after smoking marijuana cigarettes containing 2.57 percent delta9-THC. However, Fant et al. (1998) showed that the morning after exposure to 1.8 percent or 3.6 percent smoked delta9-THC, subjects had minimal residual alterations in subjective or performance measures.
A number of factors may influence marijuana's behavioral effects including the duration of use (chronic or short term), frequency of use (daily, weekly, or occasionally), and amount of use (heavy or moderate). Researchers also have examined how long behavioral impairments last following chronic marijuana use. These studies used self-reported histories of past duration, frequency, and amount of past marijuana use, and administered a variety of performance and cognitive measures at different time points following marijuana abstinence. In chronic marijuana users, behavioral impairments may persist for up to 28 days of abstinence. Solowij et al. (2002) demonstrated that after 17 hours of abstinence, 51 adult heavy chronic marijuana users performed worse on memory and attention tasks than 33 non-using controls or 51 heavy, short-term users. Another study noted that heavy, frequent marijuana users, abstinent for at least 24 hours, performed significantly worse than the controls on verbal memory and psychomotor speed tests (Messinis et al., 2006). Additionally, after at least 1 week of abstinence, young adult frequent marijuana users, aged 18-28, showed deficits in psychomotor speed, sustained attention, and cognitive inhibition (Lisdahl and Price, 2012). Adult heavy, chronic marijuana users showed deficits on memory tests after 7 days of supervised abstinence (Pope et al., 2002). However, when these same individuals were again tested after 28 days of abstinence, they did not show significant memory deficits. The authors concluded, "cannabis-associated cognitive deficits are reversible and related to recent cannabis exposure, rather than irreversible and related to cumulative lifetime use."[7] However, other researchers reported neuropsychological deficits in memory, executive functioning, psychomotor speed and manual dexterity in heavy marijuana users abstinent for 28 days (Bolla et al., 2002). Furthermore, a follow-up study of heavy marijuana users noted decision-making deficits after 25 days of supervised abstinence. (Bolla et al., 2005). However, moderate marijuana users did not show decision-making deficits after 25 days of abstinence, suggesting the amount of marijuana use may impact the duration of residual impairment.
The effects of chronic marijuana use do not seem to persist after more than 1 to 3 months of abstinence. After 3 months of abstinence, any deficits observed in IQ, immediate memory, delayed memory, and information-processing speeds following heavy marijuana use compared to pre-drug use scores were no longer apparent (Fried et al., 2005). Marijuana did not appear to have lasting effects on performance of a comprehensive neuropsychological battery when 54 monozygotic male twins (one of whom used marijuana, one of whom did not) were compared 1-20 years after cessation of marijuana use (Lyons et al., 2004). Similarly, following abstinence for a year or more, both light and heavy adult marijuana users did not show deficits on scores of verbal memory compared to non-using controls (Tait et al., 2011). According to a recent meta-analysis looking at non-acute and long-lasting effects of marijuana use on neurocognitive performance, any deficits seen within the first month following abstinence are generally not present after about 1 month of abstinence (Schreiner and Dunn, 2012).
Another aspect that may be a critical factor in the intensity and persistence of impairment resulting from chronic marijuana use is the age of first use. Individuals with a diagnosis of marijuana misuse or dependence who were seeking treatment for substance use, who initiated marijuana use before the age of 15 years, showed deficits in performance on tasks assessing sustained attention, impulse control, and general executive functioning compared to non-using controls. These deficits were not seen in individuals who initiated marijuana use after the age of 15 years (Fontes et al., 2011). Similarly, heavy, chronic marijuana users who began using marijuana before the age of 16 years had greater decrements in executive functioning tasks than heavy, chronic marijuana users who started using after the age of 16 years and non-using controls (Gruber et al., 2012). Additionally, in a prospective longitudinal birth cohort study of 1,037 individuals, marijuana dependence or chronic marijuana use was associated with a decrease in IQ and general neuropsychological performance compared to pre-marijuana exposure levels in adolescent onset users (Meier et al., 2012). The decline in adolescent-onset user's IQ persisted even after reduction or abstinence of marijuana use for at least 1 year. In contrast, the adult-onset chronic marijuana users showed no significant changes in IQ compared to pre-exposure Start Printed Page 53696 levels whether they were current users or abstinent for at least 1 year (Meier et al., 2012).
In addition to the age of onset of use, some evidence suggests that the amount of marijuana used may relate to the intensity of impairments. In the above study by Gruber et al. (2012), where early-onset users had greater deficits than late-onset users, the early-onset users reported using marijuana twice as often and using three times as much marijuana per week than the late-onset users. Meier et al. (2012) showed that the deficits in IQ seen in adolescent-onset users increased with the amount of marijuana used. Moreover, when comparing scores for measures of IQ, immediate memory, delayed memory, and information-processing speeds to pre-drug-use levels, the current, heavy, chronic marijuana users showed deficits in all three measures while current, occasional marijuana users did not (Fried et al., 2005).
Behavioral Effects of Prenatal Exposure
Studies with children at different stages of development are used to examine the impact of prenatal marijuana exposure on performance in a series of cognitive tasks. However, many pregnant women who reported marijuana use were more likely to also report use of alcohol, tobacco, and cocaine (Goldschmidt et al., 2008). Thus, with potential exposure to multiple drugs, it is difficult to determine the specific impact of prenatal marijuana exposure.
Most studies assessing the behavioral effects of prenatal marijuana exposure included women who, in addition to using marijuana, also reported using alcohol and tobacco. However, some evidence suggests an association between heavy prenatal marijuana exposure and deficits in some cognitive domains. In both 4-year-old and 6-year-old children, heavy prenatal marijuana use is negatively associated with performance on tasks assessing memory, verbal reasoning, and quantitative reasoning (Fried and Watkinson, 1987; Goldschmidt et al., 2008). Additionally, heavy prenatal marijuana use is associated with deficits in measures of sustained attention in children at the ages of 6 years and 13-16 years (Fried et al., 1992; Fried, 2002). In 9- to 12-year-old children, prenatal marijuana exposure is negatively associated with executive functioning tasks that require impulse control, visual analysis, and hypothesis (Fried et al., 1998).
Association of Marijuana Use With Psychosis
This analysis evaluates only the evidence for a direct link between prior marijuana use and the subsequent development of psychosis. Thus, this discussion does not consider issues such as whether marijuana's transient effects are similar to psychotic symptoms in healthy individuals or exacerbate psychotic symptoms in individuals already diagnosed with schizophrenia.
Extensive research has been conducted to investigate whether exposure to marijuana is associated with the development of schizophrenia or other psychoses. Although many studies are small and inferential, other studies in the literature use hundreds to thousands of subjects. At present, the available data do not suggest a causative link between marijuana use and the development of psychosis (Minozzi et al., 2010). Numerous large, longitudinal studies show that subjects who used marijuana do not have a greater incidence of psychotic diagnoses compared to those who do not use marijuana (Fergusson et al., 2005; Kuepper et al., 2011; Van Os et al., 2002).
When analyzing the available evidence of the connection between psychosis and marijuana, it is critical to determine whether the subjects in the studies are patients who are already diagnosed with psychosis or individuals who demonstrate a limited number of symptoms associated with psychosis without qualifying for a diagnosis of the disorder. For example, instead of using a diagnosis of psychosis, some researchers relied on non-standard methods of representing symptoms of psychosis including "schizophrenic cluster" (Maremmani et al., 2004), "subclinical psychotic symptoms" (Van Gastel et al., 2012), "pre-psychotic clinical high risk" (Van der Meer et al., 2012), and symptoms related to "psychosis vulnerability" (Griffith-Lendering et al., 2012). These groupings do not conform to the criteria in the Diagnostic and Statistical Manual (DSM-5) or the International Classification of Diseases (ICD-10) for a diagnosis of psychosis. Thus, these groupings are not appropriate for use in evaluating marijuana's impact on the development of actual psychosis. Accordingly, this analysis includes only those studies that use subjects diagnosed with a psychotic disorder.
In the largest study evaluating the link between psychosis and drug use, 274 of the approximately 45,500 Swedish conscripts in the study population (<0.01 percent) received a diagnosis of schizophrenia within the 14-year period following military induction from 1969 to 1983 (Andreasson et al., 1987). Of the conscripts diagnosed with psychosis, 7.7 percent (21 of the 274 conscripts with psychosis) had used marijuana more than 50 times at induction, while 72 percent (197 of the 274 conscripts with psychosis) had never used marijuana. Although high marijuana use increased the relative risk for schizophrenia to 6.0, the authors note that substantial marijuana use history "accounts for only a minority of all cases" of psychosis (Andreasson et al., 1987). Instead, the best predictor for whether a conscript would develop psychosis was a non-psychotic psychiatric diagnosis upon induction. The authors concluded that marijuana use increased the risk for psychosis only among individuals predisposed to develop the disorder. In addition, a 35-year follow up to this study reported very similar results (Manrique-Garcia et al., 2012). In this follow up study, 354 conscripts developed schizophrenia; of these 354 conscripts, 32 used marijuana more than 50 times at induction (9 percent, an odds ratio of 6.3), while 255 had never used marijuana (72 percent).
Additionally, the conclusion that the impact of marijuana may manifest only in individuals likely to develop psychotic disorders has been shown in many other types of studies. For example, although evidence shows that marijuana use may precede the presentation of symptoms in individuals later diagnosed with psychosis (Schimmelmann et al., 2011), most reports conclude that prodromal symptoms of schizophrenia appear prior to marijuana use (Schiffman et al., 2005). Similarly, a review of the gene-environment interaction model for marijuana and psychosis concluded that some evidence supports marijuana use as a factor that may influence the development of psychosis, but only in those individuals with psychotic liability (Pelayo-Teran et al., 2012).
A similar conclusion was drawn when the prevalence of schizophrenia was modeled against marijuana use across eight birth cohorts in Australia in individuals born between the years 1940 to 1979 (Degenhardt et al., 2003). Although marijuana use increased over time in adults born during the four-decade period, there was not a corresponding increase in diagnoses for psychosis in these individuals. The authors conclude that marijuana may precipitate schizophrenic disorders only in those individuals who are vulnerable to developing psychosis. Thus, marijuana per se does not appear to Start Printed Page 53697 induce schizophrenia in the majority of individuals who have tried or continue to use marijuana. However, in individuals with a genetic vulnerability for psychosis, marijuana use may influence the development of psychosis.
Cardiovascular and Autonomic Effects
Single smoked or oral doses of delta9-THC produce tachycardia and may increase blood pressure (Capriotti et al., 1988; Benowitz and Jones, 1975). Some evidence associates the tachycardia produced by delta9-THC with excitation of the sympathetic and depression of the parasympathetic nervous systems (Malinowska et al., 2012). During chronic marijuana ingestion, a tolerance to tachycardia develops (Malinowska et al., 2012).
However, prolonged delta9-THC ingestion produces bradycardia and hypotension (Benowitz and Jones, 1975). Plant-derived cannabinoids and endocannabinoids elicit hypotension and bradycardia via activation of peripherally-located CB1 receptors (Wagner et al., 1998). Specifically, the mechanism of this effect is through presynaptic CB1 receptor-mediated inhibition of norepinephrine release from peripheral sympathetic nerve terminals, with possible additional direct vasodilation via activation of vascular cannabinoid receptors (Pacher et al., 2006). In humans, tolerance can develop to orthostatic hypotension (Jones, 2002; Sidney, 2002) possibly related to plasma volume expansion, but tolerance does not develop to the supine hypotensive effects (Benowitz and Jones, 1975). Additionally, electrocardiographic changes are minimal, even after large cumulative doses of delta9-THC are administered. (Benowitz and Jones, 1975).
Marijuana smoking by individuals, particularly those with some degree of coronary artery or cerebrovascular disease, poses risks such as increased cardiac work, catecholamines and carboxyhemoglobin, myocardial infarction, and postural hypotension (Benowitz and Jones, 1981; Hollister, 1988; Mittleman et al., 2001; Malinowska et al., 2012).
Respiratory Effects
After acute exposure to marijuana, transient bronchodilation is the most typical respiratory effect (Gong et al., 1984). A recent 20-year longitudinal study with over 5,000 individuals collected information on the amount of marijuana use and pulmonary function data at years 0, 2, 5, 10, and 20 (Pletcher et al., 2012). Among the more than 5,000 individuals who participated in the study, almost 800 of them reported current marijuana use but not tobacco use at the time of assessment. Pletcher et al. (2012) found that the occasional use of marijuana is not associated with decreased pulmonary function. However, some preliminary evidence suggests that heavy marijuana use may be associated with negative pulmonary effects (Pletcher et al., 2012). Long-term use of marijuana can lead to chronic cough and increased sputum, as well as an increased frequency of chronic bronchitis and pharyngitis. In addition, pulmonary function tests reveal that large-airway obstruction can occur with chronic marijuana smoking, as can cellular inflammatory histopathological abnormalities in bronchial epithelium (Adams and Martin 1996; Hollister 1986).
Evidence regarding marijuana smoking leading to cancer is inconsistent, as some studies suggest a positive correlation while others do not (Lee and Hancox, 2011; Tashkin, 2005). Several lung cancer cases have been reported in young marijuana users with no tobacco smoking history or other significant risk factors (Fung et al., 1999). Marijuana use may dose-dependently interact with mutagenic sensitivity, cigarette smoking, and alcohol use to increase the risk of head and neck cancer (Zhang et al., 1999). However, in a large study with 1,650 subjects, a positive association was not found between marijuana and lung cancer (Tashkin et al., 2006). This finding remained true, regardless of the extent of marijuana use, when controlling for tobacco use and other potential confounding variables. Overall, new evidence suggests that the effects of marijuana smoking on respiratory function and carcinogenicity differ from those of tobacco smoking (Lee and Hancox, 2011).
Endocrine System
Experimental marijuana administration to humans does not consistently alter many endocrine parameters. In an early study, male subjects who experimentally received smoked marijuana showed a significant depression in luteinizing hormone and a significant increase in cortisol (Cone et al., 1986). However, two later studies showed no changes in hormones. Male subjects experimentally exposed to smoked delta9-THC (18 mg/marijuana cigarette) or oral delta9-THC (10 mg three times per day for 3 days and on the morning of the fourth day) showed no changes in plasma adrenocorticotropic hormone (ACTH), cortisol, prolactin, luteinizing hormone, or testosterone levels (Dax et al., 1989). Similarly, a study with 93 men and 56 women showed that chronic marijuana use did not significantly alter concentrations of testosterone, luteinizing hormone, follicle stimulating hormone, prolactin, or cortisol (Block et al., 1991). Additionally, chronic marijuana use did not affect serum levels of thyrotropin, thyroxine, and triiodothyronine (Bonnet, 2013). However, in a double-blind, placebo-controlled, randomized clinical trial of HIV-positive men, smoking marijuana dose-dependently increased plasma levels of ghrelin and leptin, and decreased plasma levels of peptide YY (Riggs et al., 2012).
The effects of marijuana on female reproductive system functionality differ between humans and animals. In monkeys, delta9-THC administration suppressed ovulation (Asch et al., 1981) and reduced progesterone levels (Almirez et al., 1983). However, in women, smoked marijuana did not alter hormone levels or the menstrual cycle (Mendelson and Mello, 1984). Brown and Dobs (2002) suggest that the development of tolerance in humans may be the cause of the discrepancies between animal and human hormonal response to cannabinoids.
The presence of in vitro delta9-THC reduces binding of the corticosteroid, dexamethasone, in hippocampal tissue from adrenalectomized rats, suggesting an interaction with the glucocorticoid receptor (Eldridge et al., 1991). Although acute delta9-THC presence releases corticosterone, tolerance develops in rats with chronic administration (Eldridge et al., 1991).
Some studies support a possible association between frequent, long-term marijuana use and increased risk of testicular germ cell tumors (Trabert et al., 2011). On the other hand, recent data suggest that cannabinoid agonists may have therapeutic value in the treatment of prostate cancer, a type of carcinoma in which growth is stimulated by androgens. Research with prostate cancer cells shows that the mixed CB1/CB2 agonist, WIN-55212-2, induces apoptosis in prostate cancer cells, as well as decreases the expression of androgen receptors and prostate-specific antigens (Sarfaraz et al., 2005).
Immune System
Cannabinoids affect the immune system in many different ways. Synthetic, natural, and endogenous cannabinoids often cause different effects in a dose-dependent biphasic manner (Croxford and Yamamura, 2005; Tanasescu and Constantinescu, 2010).
Studies in humans and animals give conflicting results about cannabinoid Start Printed Page 53698 effects on immune functioning in subjects with compromised immune systems. Abrams et al. (2003) investigated marijuana's effect on immunological functioning in 62 AIDS patients taking protease inhibitors. Subjects received one of the following three times a day: A smoked marijuana cigarette containing 3.95 percent delta9-THC, an oral tablet containing delta9-THC (2.5 mg oral dronabinol), or an oral placebo. The results showed no changes in CD4+ and CD8+ cell counts, HIV RNA levels, or protease inhibitor levels between groups. Thus, the use of cannabinoids showed no short-term adverse virologic effects in individuals with compromised immune systems. However, these human data contrast with data generated in immunodeficient mice, which demonstrated that exposure to delta9-THC in vivo suppresses immune function, increases HIV co-receptor expression, and acts as a cofactor to enhance HIV replication (Roth et al., 2005).
3. The State of Current Scientific Knowledge Regarding the Drug or Other Substance
Under the third factor, the Secretary must consider the state of current scientific knowledge regarding marijuana. Thus, this section discusses the chemistry, human pharmacokinetics, and medical uses of marijuana.
Chemistry
Marijuana is one of the common names of Cannabis sativa L. in the family Cannabaceae.
Cannabis is one of the oldest cultivated crops, providing a source of fiber, food, oil, and drug. Botanists still debate whether Cannabis should be considered as a single (The Plant List, 2010) or three species, i.e., C. sativa, C. indica, and C. ruderalis (Hillig, 2005). Specifically, marijuana is developed as sativa and indica cultivated varieties (strains) or various hybrids.
The petition defines marijuana as including all Cannabis cultivated strains. Different marijuana samples derived from various cultivated strains may have very different chemical constituents including delta9 -THC and other cannabinoids (Appendino et al., 2011). As a consequence, marijuana products from different strains will have different safety, biological, pharmacological, and toxicological profiles. Thus, all Cannabis strains cannot be considered together because of the varying chemical constituents between strains.
Marijuana contains numerous naturally occurring constituents including cannabinoids. Overall, various Cannabis strains contain more than 525 identified natural constituents. Among those constituents, the most important ones are the 21 (or 22) carbon terpenoids found in the plant, as well as their carboxylic acids, analogues, and transformation products, known as cannabinoids (Agurell et al., 1984, 1986; Mechoulam, 1973; Appendino et al., 2011). Thus far, more than 100 compounds classified as cannabinoids have been characterized (ElSohly and Slade, 2005; Radwan, ElSohly et al., 2009; Appendino et al. 2011).
Cannabinoids primarily exist in Cannabis, and published data suggest that most major cannabinoid compounds occurring naturally have been chemically identified. New and minor cannabinoids and other new compounds are continuously being characterized (Pollastro et al., 2011). So far, only two cannabinoids (cannabigerol and its corresponding acid) have been obtained from a non-Cannabis source. A South African Helichrysum (H umbraculigerum) accumulates these compounds (Appendino et al. 2011).
Among the cannabinoids found in marijuana, delta9-THC (alternate name delta1-THC) and delta-8-tetrahydrocannibinol (delta8-THC, alternate name delta6-THC) produce marijuana's characteristic psychoactive effects. Because delta9-THC is more abundant than delta8-THC, marijuana's psychoactivity is largely attributed to the former. Only a few varieties of marijuana analyzed contain delta8-THC at significant amounts (Hively et al., 1966). Delta9-THC is an optically active resinous substance, insoluble in water, and extremely lipid soluble. Chemically, delta9-THC is (6aR-trans)-6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-6H-dibenzo-[b,d]pyran-l-ol, or (-)-delta9-(trans)-tetrahydrocannabinol. The (-)-trans isomer of delta9-THC is pharmacologically 6-100 times more potent than the (+)-trans isomer (Dewey et al., 1984).
Other cannabinoids present in marijuana include CBD, CBC, and CBN. CBD, a major cannabinoid of marijuana, is insoluble in water and lipid-soluble. Chemically, CBD is 2-[(1R,6R)-3-methyl-6-prop-1-en-2-ylcyclohex-2-en-1-yl]-5-pentylbenzene-1,3-diol. CBD does not have cannabinol-like psychoactivity (Adams and Martin, 1996; Agurell et al., 1984, 1986; Hollister, 1986). CBC is another major cannabinoid in marijuana. Chemically, CBC is 2-methyl-2-(4-methylpent-3-enyl)-7-pentyl-5-chromenol. CBN, a major metabolite of delta9-THC, is also a minor naturally-occurring cannabinoid with weak psychoactivity. Chemically, CBN is 6,6,9-trimethyl-3-pentyl-benzo[c]chromen-1-ol.
Different marijuana samples derived from various cultivated strains may differ in chemical constituents including delta9-THC and other cannabinoids (Appendino et al. 2011). As a consequence, marijuana products from different strains may have different safety, biological, pharmacological, and toxicological profiles. In addition to differences between cultivated strains, the concentration of delta9-THC and other cannabinoids in marijuana may vary with growing conditions and processing after harvest. In addition to genetic differences among Cannabis species, the plant parts collected—for example, flowers, leaves, and stems—can influence marijuana's potency, quality, and purity (Adams and Martin, 1996; Agurell et al., 1984; Mechoulam, 1973). All these variations produce marijuana with potencies, as indicated by cannabinoid content, on average from as low as 1-2 percent to as high as 17 percent.
Overall, these variations in the concentrations of cannabinoids and other chemical constituents in marijuana complicate the interpretation of clinical data using marijuana. The lack of consistent concentrations of delta9 -THC and other substances in marijuana from diverse sources makes interpreting the effect of different marijuana constituents difficult. In addition to different cannabinoid concentrations having different pharmacological and toxicological profiles, the non-cannabinoid components in marijuana, such as other terpenoids and flavonoids, might also contribute to the overall pharmacological and toxicological profiles of various marijuana strains and products derived from those strains.
The term marijuana is often used to refer to a mixture of the dried flowering tops and leaves from Cannabis. Marijuana in this limiting definition is one of three major derivatives sold as separate illicit products, which also include hashish and hash oil. According to the DEA, Cannabis saliva is the primary species of Cannabis currently marketed illegally in the United States.
Marijuana can vary in cannabinoid content and potency (Agurell et al., 1984, 1986; Mechoulam 1973, Cascini et al., 2012). In the usual mixture of leaves and stems distributed as marijuana, the concentration of delta9-THC averages over 12 percent by weight. However, specially grown and selected marijuana can contain 15 percent or greater delta9-THC (Appendino et al., 2011). Thus, a 1-gram marijuana cigarette might Start Printed Page 53699 contain delta9-THC in a range from as little as 3 milligrams to as much as 150 milligrams or more. Additionally, a recent systematic review and meta-analysis found that marijuana's delta9-THC content has increased significantly from 1979-2009 (Cascini et al., 2012). In addition to smoking marijuana, individuals ingest marijuana through food made with butter or oil infused with marijuana and its extracts. These marijuana butters are generally made by adding marijuana to butter and heating it. The resultant butter is then used to cook a variety of foods. There are no published studies measuring the concentrations of cannabinoids in these marijuana food products.
Hashish consists of the dried and compressed cannabinoid-rich resinous material of Cannabis and comes in a variety of forms (e.g. balls and cakes). Individuals may break off pieces, place it into a pipe and smoke it. DEA reports that cannabinoid content in hashish averages six percent (DEA, 2005). With the development and cultivation of more high potency Cannabis strains, the average cannabinoid content in hashish will likely increase.
Hash oil is produced by solvent extraction of the cannabinoids from plant material. The extract's color and odor vary, depending on the solvent type used. Hash oil is a viscous brown- or amber-colored liquid containing approximately 50 percent cannabinoids. One or two drops of the liquid placed on a cigarette purportedly produce the equivalent of a single marijuana cigarette (DEA, 2005).
In conclusion, marijuana has hundreds of cultivars containing variable concentrations of delta[9] -THC, cannabinoids, and other compounds. Thus, marijuana is not a single chemical with a consistent and reproducible chemical profile or predictable and consistent clinical effects. A guidance for industry, entitled Botanical Drug Products, [8] provides information on the approval of botanical drug products. To investigate marijuana for medical use in a manner acceptable as support for marketing approval under an NDA, clinical studies under an IND of consistent batches of a particular marijuana product for particular disease indications should be conducted. In addition, information and data regarding the marijuana product's chemistry, manufacturing and control, pharmacology, and animal toxicology data, among others must be provided and meet the requirements for new drug approval (See 21 CFR 314.50).
Human Pharmacokinetics
Marijuana can be taken in a variety of formulations by multiple routes of administration. Individuals smoke marijuana as a cigarette, weighing between 0.5 and 1.0 gram, or in a pipe. Additionally, individuals take marijuana orally in foods or as an extract in ethanol or other solvents. More recently, access to vaporizers provides another means for abusers to inhale marijuana,
The absorption, metabolism, and pharmacokinetic profile of delta9-THC, cannabinoids, and drug products containing delta9-THC vary with route of administratfon and formulation (Adams and Martin, 1996; Agurell et al., 1984, 1986).
Pharmacokinetics of Smoked Administration of Cannabinoids
Characterization of the pharmacokinetics of delta9-THC and other cannabinoids from smoked marijuana is difficult because a subject's smoking behavior during an experiment varies (Agurell et al., 1986; Heming et al., 1986; Huestis et al., 1992a). Each puff delivers a discrete dose of delta9-THC. An experienced marijuana smoker can titrate and regulate the dose to obtain the desired acute psychological effects and minimize undesired effects. For example, under naturalistic conditions, users hold marijuana smoke in their lungs for an extended period of time which causes prolonged absorption and increases psychoactive effects. The effect of experience in the psychological response may explain why delta9-THC venous blood levels correlate poorly with intensity of effects and intoxication level (Agurell et al. 1986; Barnett et al. 1985; Huestis et al., 1992a). Puff and inhalation volumes should be recorded in studies as the concentration (dose) of cannabinoids administered can vary at different stages of smoking.
Smoked marijuana results in absorption of delta9-THC in the form of an aerosol within seconds. Psychoactive effects occur immediately following absorption, with mental and behavioral effects measurable for up to 6 hours (Grotenhermen, 2003; Hollister 1986, 1988). Delta9-THC is delivered to the brain rapidly and efficiently as expected of a very lipid soluble drug.
The bioavailability of the delta9-THC, from marijuana in a cigarette or pipe, can range from 1 to 24 percent with the fraction absorbed rarely exceeding 10 to 20 percent (Agurell et al.,1986; Hollister, 1988). The relatively low and variable bioavailability results from significant loss of delta9-THC in side-stream smoke, variation in individual smoking behaviors, cannabinoid pyrolysis, incomplete absorption of inhaled smoke, and metabolism in the lungs. An individual's experience and technique with smoking marijuana also determines the dose absorbed (Heming et al., 1986; Johansson et al., 1989). After smoking, delta9-THC venous levels decline precipitously within minutes, and continue to go down to about 5 to 10 percent of the peak level within an hour (Agurell et al., 1986, Huestis et al.,1992a, 1992b).
Pharmacokinetics for Oral Administration of Cannabinoids
After oral administration of delta9-THC or marijuana, the onset of effects starts within 30 to 90 minutes, reaches its peak after 2 to 3 hours and then remains for 4 to 12 hours (Grotenhermen, 2003; Adams and Martin, 1996; Agurell et al., 1984, 1986). Due to the delay in onset of effects, users have difficulty in titrating oral delta9-THC doses compared to smoking marijuana. Oral bioavailability of delta9-THC, whether pure or in marijuana, is low and extremely variable, ranging between 5 and 20 percent (Agurell et al., 1984, 1986). Following oral administration of radioactive-labeled delta9-THC, delta9-THC plasma levels are low relative to plasma levels after smoking or intravenous administration. Inter- and intra-subject variability occurs even with repeated dosing under controlled conditions. The low and variable oral bioavailability of delta9-THC is a consequence of its first-pass hepatic elimination from blood and erratic absorption from stomach and bowel.
Cannabinoid Metabolism and Excretion
Cannabinoid metabolism is complex. Delta9-THC is metabolized via microsomal hydroxylation to both active and inactive metabolites (Lemberger et al., 1970, 1972a, 1972b; Agurell et al., 1986; Hollister, 1988). The primary active metabolite of delta9-THC following oral ingestion is 11-hydroxy-delta9-THC. This metabolite is approximately equipotent to delta9-THC in producing marijuana-like subjective effects (Agurell et al., 1986, Lemberger and Rubin, 1975). After oral administration, metabolite levels may exceed that of delta9-THC and thus contribute greatly to the pharmacological effects of oral delta9-THC or marijuana.
Plasma clearance of delta9-THC approximates hepatic blood flow at about 950 ml/min or greater. The rapid disappearance of delta9-THC from blood Start Printed Page 53700 is largely due to redistribution to other tissues in the body, rather than to metabolism (Agurell et al., 1984, 1986). Metabolism in most tissues is relatively slow or absent. Slow release of delta9-THC and other cannabinoids from tissues and subsequent metabolism results in a long elimination half-life. The terminal half-life of delta9-THC ranges from approximately 20 hours to as long as 10 to 13 days, though reported estimates vary as expected with any slowly cleared substance and the use of assays with variable sensitivities (Hunt and Jones, 1980). Lemberger et al. (1970) determined the half-life of delta9-THC to range from 23 to 28 hours in heavy marijuana users to 60 to 70 hours in naive users. In addition to 11-hydroxy-delta9-THC, some inactive carboxy metabolites have terminal half-lives of 50 hours to 6 days or more. The latter substances serve as long-term markers in urine tests for earlier marijuana use.
The majority of the absorbed delta9-THC dose is eliminated in feces, and about 33 percent in urine. Delta9-THC enters enterohepatic circulation and undergoes hydroxylation and oxidation to 11-nor-9-carboxy-delta9-THC. The glucuronide is excreted as the major urine metabolite along with about 18 non-conjugated metabolites. Frequent and infrequent marijuana users metabolize delta9-THC similarly (Agurell et al., 1986).
Status of Research Into the Medical Uses for Marijuana
State-level public initiatives, including laws and referenda in support of the medical use of marijuana, have generated interest in the medical community and the need for high quality clinical investigation as well as comprehensive safety and effectiveness data. In order to address the need for high quality clinical investigations, the state of California established the Center for Medicinal Cannabis Research (CMCR, www.cmcr.ucsd.edu) in 2000 "in response to scientific evidence for therapeutic possibilities of cannabis[9] and local legislative initiatives in favor of compassionate use" (Grant, 2005). State legislation establishing the CMCR called for high quality medical research that would "enhance understanding of the efficacy and adverse effects of marijuana as a pharmacological agent," but stressed the project "should not be construed as encouraging or sanctioning the social or recreational use of marijuana." The CMCR funded many of the published studies on marijuana's potential use for treating multiple sclerosis, neuropathic pain, appetite suppression and cachexia. However, aside from the data produced by CMCR, no state-level medical marijuana laws have produced scientific data on marijuana's safety and effectiveness.
FDA approves medical use of a drug following a submission and review of an NDA or BLA. The FDA has not approved any drug product containing marijuana for marketing. Even so, results of small clinical exploratory studies have been published in the current medical literature. Many studies describe human research with marijuana in the United States under FDA-regulated IND applications.
However, FDA approval of an NDA is not the only means through which a drug can have a currently accepted medical use in treatment in the United States. In general, a drug may have a "currently accepted medical use" in treatment in the United States if the drug meets a five-part test. Established case law (Alliance for Cannabis Therapeutics v. DEA, 15 F.3d 1131, 1135 (D.C. Cir. 1994)) upheld the Administrator of DEA's application of the five-part test to determine whether a drug has a "currently accepted medical use." The following describes the five elements that characterize "currently accepted medical use" for a drug:[10]
i. the drug's chemistry must be known and reproducible.
"The substance's chemistry must be scientifically established to permit it to be reproduced into dosages which can be standardized. The listing of the substance in a current edition of one of the official compendia, as defined by section 201 G) of the Food, Drug and Cosmetic Act, 21 U.S.C. 321G), is sufficient to meet this requirement."
ii. there must be adequate safety studies.
"There must be adequate pharmacological and toxicological studies, done by all methods reasonably applicable, on the basis of which it could fairly and responsibly be concluded, by experts qualified by scientific training and experience to evaluate the safety and effectiveness of drugs, that the substance is safe for treating a specific, recognized disorder."
iii. there must be adequate and well-controlled studies proving efficacy.
"There must be adequate, well-controlled, well-designed, well-conducted, and well-documented studies, including clinical investigations, by experts qualified by scientific training and experience to evaluate the safety and effectiveness of drugs, on the basis of which it could be fairly and responsibly concluded by such experts that the substance will have the intended effect in treating a specific, recognized disorder."
iv. the drug must be accepted by qualified experts.
"The drug has a New Drug Application (NDA) approved by the Food and Drug Administration, pursuant to the Food, Drug and Cosmetic Act, 21 U.S.C. 355. Or, a consensus of the national community of experts, qualified by scientific training and experience to evaluate the safety and effectiveness of drugs, accepts the safety and effectiveness of the substance for use in treating a specific, recognized disorder. A material conflict of opinion among experts precludes a finding of consensus." and
v. the scientific evidence must be widely available.
"In the absence of NDA approval, information concerning the chemistry, pharmacology, toxicology, and effectiveness of the substance must be reported, published, or otherwise widely available, in sufficient detail to permit experts, qualified by scientific training and experience to evaluate the safety and effectiveness of drugs, to fairly and responsibly conclude the substance is safe and effective for use in treating a specific, recognized disorder."
Marijuana does not meet any of the five elements necessary for a drug to have a "currently accepted medical use."
Firstly, the chemistry of marijuana, as defined in the petition, is not reproducible in terms of creating a standardized dose. The petition defines marijuana as including all Cannabis cultivated strains. Different marijuana samples derived from various cultivated strains may have very different chemical constituents including delta9-THC and other cannabinoids (Appendino et al., 2011). As a consequence, marijuana products from different strains will have different safety, biological, pharmacological, and toxicological profiles. Thus, when considering all Cannabis strains together, because of the varying chemical constituents, reproducing consistent standardized doses is not possible. Additionally, smoking marijuana currently has not been shown to allow delivery of consistent and reproducible doses. However, if a specific Cannabis strain is grown and processed under strictly controlled conditions, the plant chemistry may be kept consistent enough to produce reproducible and standardized doses. Start Printed Page 53701
As to the second and third criteria; there are neither adequate safety studies nor adequate and well-controlled studies proving marijuana's efficacy. To support the petitioners' assertion that marijuana has accepted medical use, the petitioners cite the American Medical Association's (AMA) 2009 report entitled "Use of Cannabis for Medicinal Purposes." The petitioners claim the AMA report is evidence the AMA accepts marijuana's safety and efficacy. However, the 2009 AMA report clarifies that the report "should not be viewed as an endorsement of state-based medical cannabis programs, the legalization of marijuana, or that scientific evidence on the therapeutic use of cannabis meets the same and current standards for a prescription drug product."[11]
Currently, no published studies conducted with marijuana meet the criteria of an adequate and well-controlled efficacy study. The criteria for an adequate and well-controlled study for purposes of determining the safety and efficacy of a human drug are defined under the Code of Federal Regulations (CFR) in 21 CFR 314.126. In order to assess this element, FDA conducted a review of clinical studies published and available in the public domain before February, 2013. Studies were identified through a search of PubMed[12] for articles published from inception to February 2013, for randomized controlled trials using marijuana to assess marijuana's efficacy in any therapeutic indication. Additionally, the review included studies identified through a search of bibliographic references in relevant systematic reviews and identified studies presenting original research in any language. Selected studies needed to be placebo-controlled and double-blinded. Additionally, studies needed to encompass administered marijuana plant material. There was no requirement for any specific route of administration, nor any age limits on study subjects. Studies were excluded that used placebo marijuana supplemented by the addition of specific amounts of THC or other cannabinoids. Additionally, studies administering marijuana plant extracts were excluded.
The PubMed search yielded a total of 566 abstracts of scientific articles. Of these abstracts, a full-text review was conducted with 85 papers to assess eligibility. Of the studies identified through the search of the references and the 566 abstracts from the PubMed search, only 11 studies met all the criteria for selection (Abrams et al., 2007; Corey-Bloom et al., 2012; Crawford and Merritt, 1979; Ellis et al., 2009; Haney et al., 2005; Haney et al., 2007; Merritt et al., 1980; Tashkin et al., 1974; Ware et al., 2010; Wilsey et al., 2008; Wilsey et al., 2013). These 11 studies were published between 1974 and 2013. Ten of these studies were conducted in the United States and one study was conducted in Canada. The identified studies examine the effects of smoked and vaporized marijuana for the indications of chronic neuropathic pain, spasticity related to Multiple Sclerosis (MS), appetite stimulation in human immunodeficiency virus (HIV) patients, glaucoma, and asthma. All studies used adult subjects.
The 11 identified studies were individually evaluated to determine if they successfully meet accepted scientific standards. Specifically, they were evaluated on study design including subject selection criteria, sample size, blinding techniques, dosing paradigms, outcome measures, and the statistical analysis of the results. The analysis relied on published studies, thus information available about protocols, procedures, and results were limited to documents published and widely available in the public domain. The review found that all 11 studies that examined effects of inhaled marijuana do not currently prove efficacy of marijuana in any therapeutic indication based on a number of limitations in their study design; however, they may be considered proof of concept studies. Proof of concept studies provide preliminary evidence on a proposed hypothesis involving a drug's effect. For drugs under development, the effect often relates to a short-term clinical outcome being investigated. Proof of concept studies often serve as the link between preclinical studies and dose ranging clinical studies. Thus, proof of concept studies generally are not sufficient to prove efficacy of a drug because they provide only preliminary information about the effects of a drug.
In addition to the lack of published adequate and well-controlled efficacy studies proving efficacy, the criteria for adequate safety studies has also not been met. Importantly, in its discussion of the five-part test used to determine whether a drug has a "currently accepted medical use," DEA said, "No drug can be considered safe in the abstract. Safety has meaning only when judged against the intended use of the drug, its known effectiveness, its known and potential risks, the severity of the illness to be treated, and the availability of alternative remedies" (57 FR 10504). When determining whether a drug product is safe and effective for any indication, FDA performs an extensive risk-benefit analysis to determine whether the risks posed by the drug product's side effects are outweighed by the drug product's potential benefits for a particular indication. Thus, contrary to the petitioner's assertion that marijuana has accepted safety, in the absence of an accepted therapeutic indication which can be weighed against marijuana's risks, marijuana does not satisfy the element for having adequate safety studies such that experts may conclude that it is safe for treating a specific, recognized disorder.
The fourth of the five elements for determining "currently accepted medical use" requires that the national community of experts, qualified by scientific training and experience to evaluate the safety and effectiveness of drugs, accepts the safety and effectiveness of the substance for use in treating a specific, recognized disorder. A material conflict of opinion among experts precludes a finding of consensus. Medical practitioners who are not experts in evaluating drugs are not qualified to determine whether a drug is generally recognized as safe and effective or meets NDA requirements (57 FR 10499-10505).
There is no evidence that there is a consensus among qualified experts that marijuana is safe and effective for use in treating a specific, recognized disorder. As discussed above, there are not adequate scientific studies that show marijuana is safe and effective in treating a specific, recognized disorder. In addition, there is no evidence that a consensus of qualified experts have accepted the safety and effectiveness of marijuana for use in treating a specific, recognized disorder. Although medical practitioners are not qualified by scientific training and experience to evaluate the safety and effectiveness of drugs, we also note that the AMA's report, entitled "Use of Cannabis for Medicinal Purposes," does not accept that marijuana currently has accepted medical use. Furthermore, based on the above definition of a "qualified expert", who is an individual qualified by scientific training and experience to evaluate the safety and effectiveness of a drug, state-level medical marijuana laws do not provide evidence of a consensus among qualified experts that marijuana is safe and effective for use in treating a specific, recognized disorder. Start Printed Page 53702
As to the fifth part of the test, which requires that information concerning the chemistry, pharmacology, toxicology, and effectiveness of marijuana to be reported in sufficient detail, the scientific evidence regarding all of these aspects is not available in sufficient detail to allow adequate scientific scrutiny. Specifically, the scientific evidence regarding marijuana's chemistry in terms of a specific Cannabis strain that could produce standardized and reproducible doses is not currently available.
Alternately, a drug can be considered to have a "currently accepted medical use with severe restrictions" (21 U.S.C. 812(b)(2)(B)), as allowed under the stipulations for a Schedule II drug. Yet, as stated above, currently marijuana does not have any accepted medical use, even under conditions where its use is severely restricted.
In conclusion, to date, research on marijuana's medical use has not progressed to the point where marijuana is considered to have a "currently accepted medical use" or a "currently accepted medical use with severe restrictions."
4. Its History and Current Pattern of Abuse
Under the fourth factor, the Secretary must consider the history and current pattern of marijuana abuse. A variety of sources provide data necessary to assess abuse patterns and trends of marijuana. The data indicators of marijuana use include the NSDUH, MTF, DAWN, and TEDS. The following briefly describes each data source, and summarizes the data from each source.
National Survey on Drug Use and Health (NSDUH)13
According to 2012 NSDUH[14] data, the most recent year with complete data, the use of illicit drugs, including marijuana, is increasing. The 2012 NSDUH estimates that 23.9 million individuals over 12 years of age (9.2 percent of the U.S. population) currently use illicit drugs, which is an increase of 4.8 million individuals from 2004 when 19.1 million individuals (7.9 percent of the U.S. population) were current illicit drug users. NSDUH reports marijuana as the most commonly used illicit drug, with 18.9 million individuals (7.3 percent of the U.S. population) currently using marijuana in 2012. This represents an increase of 4.3 million individuals from 2004, when 14.6 million individuals (6.1 percent of the U.S. population) were current marijuana users.
The majority of individuals who try marijuana at least once in their lifetime do not currently use marijuana. The 2012 NSDUH estimates that 111.2 million individuals (42.8 percent of the U.S. population) have used marijuana at least once in their lifetime. Based on this estimate and the estimate for the number of individuals currently using marijuana, approximately 16.9 percent of those who have tried marijuana at least once in their lifetime currently use marijuana; conversely, 83.1 percent do not currently use marijuana. In terms of the frequency of marijuana use, an estimated 40.3 percent of individuals who used marijuana in the past month used marijuana on 20 or more days within the past month. This amount corresponds to an estimated 7.6 million individuals who used marijuana on a daily or almost daily basis.
Some characteristics of marijuana users are related to age, gender, and criminal justice system involvement. In observing use among different age cohorts, the majority of individuals who currently use marijuana are shown to be between the ages of 18-25, with 18.7 percent of this age group currently using marijuana. In the 26 and older age group, 5.3 percent of individuals currently use marijuana. Additionally, in individuals aged 12 years and older, males reported more current marijuana use than females.
NSDUH includes a series of questions aimed at assessing the prevalence of dependence and abuse of different substances in the past 12 months.[15] In 2012, marijuana was the most common illicit drug reported by individuals with past year dependence or abuse. An estimated 4.3 million individuals meet the NSDUH criteria for marijuana dependence or abuse in 2012. The estimated rates and number of individuals with marijuana dependence or abuse has remained similar from 2002 to 2012. In addition to data on dependence and abuse, NSDUH includes questions aimed at assessing treatment for a substance use problem.[16] In 2012, an estimated 957,000 persons received treatment for marijuana use during their most recent treatment in the year prior to the survey.
Monitoring the Future (MTF)17
According to MTF,[18] rates of marijuana and illicit drug use declined for all three grades from 2005 through 2007. However, starting around 2008, rates of annual use of illicit drugs and marijuana increased through 2013 for all three grades. Marijuana remained the most widely used illicit drug during all time periods. The prevalence of annual and past month marijuana use in 10th and 12th graders in 2013 is greater than in 2005. Table 1 lists the lifetime, annual, and monthly prevalence rates of various drugs for 8th, 10th, and 12th graders in 2013.
Start Printed Page 53703

Drug Abuse Warning Network (DAWN)19
Importantly, many factors can influence the estimates of ED visits, including trends in overall use of a substance as well as trends in the reasons for ED usage. For instance, some drug users may visit EDs for life-threatening issues while others may visit to seek care for detoxification because they needed certification before entering treatment. Additionally, DAWN data do not distinguish the drug responsible for the ED visit from other drugs that may have been used concomitantly. As stated in a DAWN report, "Since marijuana/hashish is frequently present in combination with other drugs, the reason for the ED visit may be more relevant to the other drug(s) involved in the episode."
For 2011, DAWN[20] estimates a total of 5,067,374 (95 percent confidence interval [CI]: 4,616,753 to 5,517,995) drug-related ED visits from the entire United States. Of these, approximately 2,462,948 ([CI]: 2,112,868 to 2,813,028) visits involved drug misuse or abuse.
During the same period, DAWN estimates that 1,252,500 (CI: 976,169 to 1,528,831) drug related ED visits involved illicit drugs. Thus, over half of all drug-related ED visits associated with drug misuse or abuse involved an illicit drug. For ED visits involving illicit drugs, 56.3 percent involved multiple drugs while 43.7 percent involved a single drug.
Marijuana was involved in 455,668 ED visits (CI: 370,995 to 540,340), while cocaine was involved in 505,224 (CI: 324,262 to 686, 185) ED visits, heroin was involved in 258,482 (CI: 205,046 to 311,918) ED visits and stimulants including amphetamine and methamphetamine were involved in 159,840 (CI: 100,199 to 219,481) ED visits. Other illicit drugs, such as PCP, MDMA, GHB and LSD were much less frequently associated with ED visits. The number of ED visits involving marijuana has increased by 62 percent since 2004.
Marijuana-related ED visits were most frequent among young adults and minors. Individuals under the age of 18 accounted for 13.2 percent of these marijuana-related visits, whereas this age group accounted for approximately 1.2 percent of ED visits involving cocaine, and less than 1 percent of ED visits involving heroin. However, the age group with the most marijuana-related ED visits was between 25 and 29 years old. Yet, because populations differ between age groups, a standardized measure for population size is useful to make comparisons. For marijuana, the rates of ED visits per 100,000 population were highest for patients aged 18 to 20 (443.8 ED visits per 100,000) and for patients aged 21 to 24 (446.9 ED visits per 100,000).
While DAWN provides estimates for ED visits associated with the use of medical marijuana for 2009-2011, the validity of these estimates is questionable. Because the drug is not approved by the FDA, reporting medical marijuana may be inconsistent and reliant on a number of factors including whether the patient self-reports the marijuana use as medicinal, how the treating health care provider records the marijuana use, and lastly how the SAMHSA coder interprets the report. All of these aspects will vary greatly between states with medical marijuana laws and states without medical marijuana laws. Thus, even though estimates are reported for medical marijuana related ED visits, medical marijuana estimates cannot be assessed with any acceptable accuracy at this time, as FDA has not approved marijuana treatment of any medical condition. These data show the difficulty in evaluating abuse of a product that is not currently approved by FDA, but authorized for medical use, albeit inconsistently, at the state level. Thus, we believe the likelihood of the treating health care provider or SAMHSA coder attributing the ED visit to "medical marijuana" versus "marijuana" to be very low. Overall, the available data are inadequate to characterize its abuse at the community level. Start Printed Page 53704
Treatment Episode Data Set (TEDS)21
Primary marijuana abuse accounted for 18.1 percent of all 2011 TEDS[22] admissions. Individuals admitted for primary marijuana abuse were nearly three-quarters (73.4 percent) male, and almost half (45.2 percent) were white. The average age at admission was 24 years old, and 31.1 percent of individuals admitted for primary marijuana abuse were under the age of 18. The reported frequency of marijuana use was 24.3 percent reporting daily use. Almost all (96.8 percent) primary marijuana users utilized the substance by smoking. Additionally, 92.9 percent reported using marijuana for the first time before the age of 18.
An important aspect of TEDS admission data for marijuana is of the referral source for treatment. Specifically, primary marijuana admissions were less likely than all other admissions to either be self-referred or referred by an individual for treatment. Instead, the criminal justice system referred more than half (51.6 percent) of primary marijuana admissions.
Since 2003, the percent of admissions for primary marijuana abuse increased from 15.5 percent of all admissions in 2003 to 18.1 percent in 2011. This increase is less than the increase seen for admissions for primary opioids other than heroin, which increased from 2.8 percent in 2003 to 7.3 percent in 2011. In contrast, the admissions for primary cocaine abuse declined from 9.8 percent in 2003 to 2.0 percent in 2011.
5. The Scope, Duration, and Significance of Abuse
Under the fifth factor, the Secretary must consider the scope, duration, and significance of marijuana abuse. According to 2012 data from NSDUH and 2013 data from MTF, marijuana remains the most extensively used illegal drug in the United States, with 42.8 percent of U.S. individuals over age 12 (111.2 million) and 45.5 percent of 12th graders having used marijuana at least once in their lifetime. Although the majority of individuals over age 12 (83.1 percent) who have ever used marijuana in their lifetime do not use the drug monthly, 18.9 million individuals (7.3 percent of the U.S. population) report that they used marijuana within the past 30 days. An examination of use among various age cohorts through NSDUH demonstrates that monthly use occurs primarily among college-aged individuals, with use dropping off sharply after age 25. Additionally, NSDUH data show the number of individuals reporting past-month use of marijuana has increased by 4.3 million individuals since 2004. Data from MTF shows that annual prevalence of marijuana use declined for all three grades from 2005 through 2007, then began to rise through 2013. Additionally, in 2013, 1.1 percent of 8th graders, 4.0 percent of 10th graders, and 6.5 percent of 12th graders reported daily use of marijuana, defined as use on 20 or more days within the past 30 days.
The 2011 DAWN data show that marijuana use was mentioned in 455,668 ED visits, which amounts to approximately 36.4 percent of all illicit drug-related ED visits.[23]
TEDS data for 2011 show that 18.1 percent of all admissions were for primary marijuana abuse.[24] Between 2003 and 2011, there was a 2.6 percent increase in the number of TEDS admissions for primary marijuana use. Approximately 61.5 percent of primary marijuana admissions in 2011 were for individuals under the age of 25 years.
6. WHAT, if Any, Risk There Is to the Public Health
Under the sixth factor, the Secretary must consider the risks posed to the public health by marijuana. Factors 1, 4, and 5 include a. discussion of the risk to the public health as measured by emergency room episodes and drug treatment admissions. Additionally, Factor 2 includes a discussion of marijuana's central nervous system, cognitive, cardiovascular, autonomic, respiratory, and immune system effects. Factor 6 focuses on the health risks to the individual user in terms of the risks from acute and chronic use of marijuana, as well as the "gateway hypothesis."
Risks From Acute Use of Marijuana
Acute use of marijuana impairs psychomotor performance, including complex task performance, which makes operating motor vehicles or heavy equipment after using marijuana inadvisable (Ramaekers et al., 2004; Ramaekers et al., 2006a). A meta-analysis conducted by Li et al. (2011) showed an association between marijuana use by the driver and a significantly increased risk of involvement in a car accident. Additionally, in a minority of individuals who use marijuana, some potential responses include dysphoria and psychological distress, including prolonged anxiety reactions (Haney et al., 1999).
Risks From Chronic Use of Marijuana
A distinctive marijuana withdrawal syndrome following long term or chronic use has been identified. The withdrawal syndrome indicates that marijuana produces physical dependence that is mild, short-lived, and comparable to tobacco withdrawal (Budney et al., 2008). Marijuana withdrawal syndrome is described in detail below under Factor 7.
The following states how the DSM-V (2013) of the American Psychiatric Association describes the consequences of Cannabis[25] abuse:
Individuals with cannabis use disorder may use cannabis throughout the day over a period of months or years, and thus may spend many hours a day under the influence. Others may use less frequently, but their use causes recurrent problems related to family, Start Printed Page 53705 school, work, or other important activities (e.g., repeated absences at work; neglect of family obligations). Periodic cannabis use and intoxication can negatively affect behavioral and cognitive functioning and thus interfere with optimal performance at work or school, or place the individual at increased physical risk when performing activities that could be physically hazardous (e.g., driving a car; playing certain sports; performing manual work activities, including operating machinery). Arguments with spouses or parents over the use of cannabis in the home, or its use in the presence of children, can adversely impact family functioning and are common features of those with cannabis use disorder. Last, individuals with cannabis use disorder may continue using marijuana despite knowledge of physical problems (e.g., chronic cough related to smoking) or psychological problems (e.g., excessive sedation or exacerbation of other mental health problems) associated with its use.
Marijuana as a "Gateway Drug"
Kandel (1975) proposed nearly 40 years ago the hypothesis that marijuana is a "gateway drug" that leads to the use or abuse of other illicit drugs. Since that time, epidemiological research explored this premise. Overall, research does not support a direct causal relationship between regular marijuana use and other illicit drug use. The studies examining the gateway hypothesis are limited. First, in general, studies recruit individuals influenced by a myriad of social, biological, and economic factors that contribute to extensive drug abuse (Hall & Lynskey, 2005). Second, most studies that test the hypothesis that marijuana use causes abuse of illicit drugs use the determinative measure any use of an illicit drug, rather than DSM-5 criteria for drug abuse or dependence on an illicit drug (DSM-5, 2013). Consequently, although an individual who used marijuana may try other illicit drugs, the individual may not regularly use drugs, or have a diagnosis of drug abuse or dependence.
Little evidence supports the hypothesis that initiation of marijuana use leads to an abuse disorder with other illicit substances. For example, one longitudinal study of 708 adolescents demonstrated that early onset marijuana use did not lead to problematic drug use (Kandel & Chen, 2000). Similarly, Nace et al. (1975) examined Vietnam-era soldiers who extensively abused marijuana and heroin while they were in the military, and found a lack of correlation of a causal relationship demonstrating marijuana use leading to heroin addiction. Additionally, in another longitudinal study of 2,446 adolescents, marijuana dependence was uncommon but when it did occur, the common predictors of marijuana dependence were the following: Parental death, deprived socio-economic status, and baseline illicit drug use other than marijuana (von Sydow et al., 2002).
When examining the association between marijuana and illicit drugs, focusing on drug use versus abuse or dependence, different patterns emerge. For example, a study examining the possible causal relationship of the gateway hypothesis found a correlation between marijuana use in adolescents and other illicit drug use in early adulthood and, adjusting for age-linked experiences, did not effect this correlation (Van Gundy and Rebellon, 2010). However, when examining the association in terms of development of drug abuse; age-linked stressors and social roles moderated the correlation between marijuana use in adolescents and other illicit drug abuse. Similarly, Degenhardt et al. (2009) examined the development of drug dependence and found an association that did not support the gateway hypothesis. Specifically, drug dependence was significantly associated with the use of other illicit drugs prior to marijuana use.
Interestingly, the order of initiation of drug use seems to depend on the prevalence of use of each drug, which varies by country. Based on the World Health Organization (WHO) World Mental Health Survey that includes data from 17 different countries, the order of drug use initiation varies by country and relates to prevalence of drug use in each country (Degenhardt et al., 2010). Specifically, in the countries with the lowest prevalence of marijuana use, use of other illicit drugs before marijuana was common. This sequence of initiation is less common in countries with higher prevalence of marijuana use. A study of 9,282 households in the United States found that marijuana use often preceded the use of other illicit drugs; however, prior non-marijuana drug dependence was also frequently correlated with higher levels of illicit drug abuse (Degenhardt et al., 2009). Additionally, in a large 25-year longitudinal study of 1,256 New Zealand children, the author concluded that marijuana use correlated to an increased risk of abuse of other drugs, including cocaine and heroin (Fergusson et al., 2005).
Although many individuals with a drug abuse disorder may have used marijuana as one of their first illicit drugs, this fact does not correctly lead to the reverse inference that most individuals who used marijuana will inherently go on to try or become regular users of other illicit drugs. Specifically, data from the 2011 NSDUH survey illustrates this issue (SAMHSA, 2012). NSDUH data estimates 107.8 million individuals have a lifetime history of marijuana use, which indicates use on at least one occasion, compared to approximately 36 million individuals having a lifetime history of cocaine use and approximately 4 million individuals having a lifetime history of heroin use. NSDUH data do not provide information about each individual's specific drug history. However, even if one posits that every cocaine and heroin user previously used marijuana, the NSDUH data show that marijuana use at least once in a lifetime does not predict that an individual will also use another illicit drug at least once.
Finally, a review of the gateway hypothesis by Vanyukov et al. (2012) notes that because the gateway hypothesis only addresses the order of drug use initiation, the gateway hypothesis does not specify any mechanistic connections between drug "stages" following exposure to marijuana and does not extend to the risks for addiction. This concept contrasts with the concept of a common liability to addiction that involves mechanisms and biobehavioral characteristics pertaining to the entire course of drug abuse risk and disorders.
7. Its Psychic or Physiologic Dependence Liability
Under the seventh factor, the Secretary must consider marijuana's psychic or physiological dependence liability.
Psychic or psychological dependence has been shown in response to marijuana's psychoactive effects. Psychoactive responses to marijuana are pleasurable to many humans and are associated with drug-seeking and drug-taking (Maldonado, 2002). Moreover, high levels of psychoactive effects, notably positive reinforcement, are associated with increased marijuana use, abuse, and dependence (Scherrer et al., 2009; Zeiger et al., 2010). Epidemiological data support these findings through 2012 NSDUH statistics that show that of individuals years 12 or older who used marijuana in the past month, an estimated 40.3 percent used marijuana on 20 or more days within the past month. This equates to approximately 7.6 million individuals aged 12 or older who used marijuana on a daily or almost daily basis. Start Printed Page 53706 Additionally, the 2013 MTF data report the prevalence of daily marijuana use, defined as use on 20 or more days within the past 30 days, in 8th, 10th, and 12th graders is 1.1 percent, 4.0 percent, and 6.5 percent, respectively.
Tolerance is a state of adaptation where exposure to a drug induces changes that result in a diminution of one or more of the drug's effects over time (American Academy of Pain Medicine, American Pain Society and American Society of Addiction Medicine consensus document, 2001). Tolerance can develop to some, but not all, of marijuana's effects. Specifically, tolerance does not seem to develop in response to many of marijuana's psychoactive effects. This lack of tolerance may relate to electrophysiological data demonstrating that chronic delta9-THC administration does not affect increased neuronal firing in the ventral tegmental area, a region known to play a critical role in drug reinforcement and reward (Wu and French, 2000). In the absence of other abuse indicators, such as rewarding properties, the presence of tolerance or physical dependence does not determine whether a drug has abuse potential.
However, humans can develop tolerance to marijuana's cardiovascular, autonomic, and behavioral effects (Jones et al., 1981). Tolerance to some of marijuana's behavioral effects seems to develop after heavy marijuana use, but not after occasional marijuana use. For instance, following acute administration of marijuana, heavy marijuana users did not exhibit impairments in tracking and attention tasks, as were seen in occasional marijuana users (Ramaekers et al., 2009). Furthermore, a neurophysiological assessment administered through an electroencephalograph (EEG) which measures event-related potentials (ERP) conducted in the same subjects as the previous study, found a corresponding effect in the P100[26] component of ERPs. Specifically, corresponding to performance on tracking and attention tasks, heavy marijuana users showed no changes in P100 amplitudes following acute marijuana administration, although occasional users showed a decrease in P100 amplitudes (Theunissen et al., 2012). A possible mechanism underlying tolerance to marijuana's effects may be the down-regulation of cannabinoid receptors (Hirvonen et al., 2012; Gonzalez et al., 2005; Rodriguez de Fonseca et al., 1994; Oviedo et al., 1993).
Importantly, pharmacological tolerance alone does not indicate a drug's physical dependence liability. In order for physical dependence to exist, evidence of a withdrawal syndrome is needed. Physical dependence is a state of adaptation, manifested by a drug-class specific withdrawal syndrome produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist (ibid). Many medications not associated with abuse or addiction can produce physical dependence and withdrawal symptoms after chronic use.
Discontinuation of heavy, chronic marijuana use has been shown to lead to physical dependence and withdrawal symptoms (American Psychiatric Association DSM-V, 2013; Budney and Hughes, 2006; Haney et al., 1999). In heavy, chronic marijuana users, the most commonly reported withdrawal symptoms are sleep difficulties, decreased appetite or weight loss, irritability, anger, anxiety or nervousness, and restlessness. Some less commonly reported withdrawal symptoms are depressed mood, sweating, shakiness, physical discomfort, and chills (Budney and Hughes, 2006; Haney et al., 1999). The occurrence of marijuana withdrawal symptoms in light or non-daily marijuana users has not been established. The American Psychiatric Association's DSM-V (2013) includes a list of symptoms of "cannabis withdrawal." Most marijuana withdrawal symptoms begin within 24-48 hours of discontinuation, peak within 4-6 days, and last for 1-3 weeks. Marijuana withdrawal syndrome has been reported in adolescents and adults admitted for substance abuse treatment.
Based on clinical descriptions, this syndrome appears to be mild compared to classical alcohol and barbiturate withdrawal syndromes, which can include more serious symptoms such as agitation, paranoia, and seizures. Multiple studies comparing marijuana and tobacco withdrawal symptoms in humans demonstrate that the magnitude and time course of the two withdrawal syndromes are similar (Budney et al., 2008; Vandrey et al., 2005, 2008).
8. Whether the Substance Is an Immediate Precursor of a Substance Already Controlled Under This Article
Under the eight factor analysis, the Secretary must consider whether marijuana is an immediate precursor of a controlled substance. Marijuana is not an immediate precursor of another controlled substance.
Recommendation
After consideration of the eight factors discussed above, FDA recommends that marijuana remain in Schedule I of the CSA. NIDA concurs with this scheduling recommendation. Marijuana meets the three criteria for placing a substance in Schedule I of the CSA under 21 U.S.C. 812(b)(l):
(1) Marijuana has a high potential for abuse:
A number of factors indicate marijuana's high abuse potential, including the large number of individuals regularly using marijuana, marijuana's widespread use, and the vast amount of marijuana available for illicit use. Approximately 18.9 million individuals in the United States (7.3 percent of the U.S. population) used marijuana monthly in 2012. Additionally, approximately 4.3 million individuals met diagnostic criteria for marijuana dependence or abuse in the year prior to the 2012 NSDUH survey. A 2013 survey indicates that by 12th grade, 36.4 percent of students report using marijuana within the past year, and 22.7 percent report using marijuana monthly. In 2011, 455,668 ED visits were marijuana-related, representing 36.4 percent of all illicit drug-related episodes. Primary marijuana use accounted for 18.1 percent of admissions to drug treatment programs in 2011. Additionally, marijuana has dose-dependent reinforcing effects, as demonstrated by data showing that humans prefer relatively higher doses to lower doses. Furthermore, marijuana use can result in psychological dependence.
(2) Marijuana has no currently accepted medical use in treatment in the United States:
FDA has not approved a marketing application for a marijuana drug product for any indication. The opportunity for scientists to conduct clinical research with marijuana exists, and there are active INDs for marijuana; however, marijuana does not have a currently accepted medical use for treatment in the United States, nor does marijuana have an accepted medical use with severe restrictions.
A drug has a "currently accepted medical use" if all of the following five elements have been satisfied:
a. the drug's chemistry is known and reproducible;
b. there are adequate safety studies;
c. there are adequate and well-controlled studies proving efficacy;
d. the drug is accepted by qualified experts; and
e. the scientific evidence is widely available.
Start Printed Page 53707
[57 FR 10499, March 26, 1992]
Marijuana does not meet any of the elements for having a "currently accepted medical use."
First, FDA broadly evaluated marijuana, and did not focus its evaluation on particular strains of marijuana or components or derivatives of marijuana. Since different strains may have different chemical constituents, marijuana, as identified in this petition, does not have a known and reproducible chemistry, which would be needed to provide standardized doses.
Second, there are not adequate safety studies on marijuana in the medical literature in relation to a specific, recognized disorder. Third, there are no published adequate and well controlled studies proving efficacy of marijuana. Fourth, there is no evidence that qualified experts accept marijuana for use in treating a specific, recognized disorder. Lastly, the scientific evidence regarding marijuana's chemistry in terms of a specific Cannabis strain that could produce standardized and reproducible doses is not currently available, so the scientific evidence on marijuana is not widely available.
Alternately, a Schedule II drug can be considered to have a "currently accepted medical use with severe restrictions" (21 U.S.C. 812(b)(2)(B)). Yet as stated above, the lack of accepted medical use for a specific, recognized disorder precludes the use of marijuana even under conditions where its use is severely restricted.
In conclusion, to date, research on marijuana's medical use has not developed to the point where marijuana is considered to have a "currently accepted medical use" or a "currently accepted medical use with severe restrictions."
(3) There is a lack of accepted safety for use of marijuana under medical supervision:
There are currently no FDA-approved marijuana drug products. Marijuana does not have a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions. Thus, FDA has not determined that marijuana is safe for use under medical supervision.
In addition, FDA cannot conclude that marijuana has an acceptable level of safety relative to its effectiveness in treating a specific, recognized disorder without evidence that the substance is contamination free, and assurance of a consistent and predictable dose. Investigations into the medical use of marijuana should include information and data regarding the chemistry, manufacturing, and specifications of marijuana. Additionally, a procedure for delivering a consistent dose of marijuana should also be developed. Therefore, FDA concludes marijuana does not currently have an accepted level of safety for use under medical supervision.
References
Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, Benowitz NL, Bredt BM, Kosel B, Aberg JA, Deeks SG, Mitchell TF, Mulligan K, Bacchetti P, McCune JM, Schambelan M. Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized, placebo controlled clinical trial. Ann Intern Med. 2003 Aug 19; 139(4):258-66.
Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, Kelly ME, Rowbotham MC, and Petersen KL. 2007. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology 68(7): 515-521.
Adams, LB., and Martin, B.R. Cannabis: Pharmacology and toxicology in animals and humans. Addiction 1996, 91(11):1585-1614.
Agurell, S., Dewey, W.L., and Willett, R.E., eds. The Cannabinoids: Chemical, Pharmacologic, and Therapeutic Aspects. New York: Academic Press, 1984.
Agurell, S.; Halldin, M.; Lindgren, J.E.; Ohlsson, A.; Widman, M.; Gillespie, H.; and Hollister, L. Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev 1986, 38(1), 21-43.
Almirez RG, Smith CG, Asch RH. The effects of marijuana extract and delta 9-tetrahydrocannabinol on luteal function in the rhesus monkey. Fertil Steril. 1983 Feb; 39(2):212-7.
Ameri, A. The effects of cannabinoids on the brain. Progress in Neurobiology 1999, 58 (4), 315-348.
American Academy of Pain Medicine, American Pain Society and American Society of Addiction Medicine Consensus Document. Definitions related to the use of opioids for the treatment of pain. 2001.
Andreasson S, Allebeck P, Engstrom A, Rydberg U. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet. 1987 Dec 26; 2(8574):1483-6.
Appendino G, Chianese G, Taglialatela-Scafati O. Cannabinoids: occurrence and medicinal chemistry. Curr Med Chem. 2011; 18(7):1085-99.
Asch RH, Smith CG, Siler-Khodr TM, Pauerstein CJ. Effects of delta 9-tetrahydrocannabinol during the follicular phase of the rhesus monkey (Macaca mulatta). J Clin Endocrinol Metab. 1981 Jan; 52(1):50-5.
Balster, R.L., Prescott, W.R., delta9-Tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci. & Biobehav. Rev. 1992, 16(1), 55-62.
Balster RL and Bigelow GE. Guidelines and methodological reviews concerning drug abuse liability assessment. Drug and Alcohol Dependence. 2003; 70: S13-S40.
Barnett, G.; Licko, V.; and Thompson, T. Behavioral pharmacokinetics of marijuana. Psychopharmacology 1985, 85(1), 51-56.
Battista N, Di TM, Bari M, Maccarrone M. The endocannabinoid system: an overview. Front.Behav.Neurosci. 2012; 6:9.
Benowitz NL, Jones RT. Cardiovascular effects of prolonged delta-9-tetrahydrocannabinol ingestion. Clin Pharmacol Ther. 1975 Sep; 18(3):287-97.
Benowitz NL, Jones RT. Cardiovascular and metabolic considerations in prolonged cannabinoid administration in man. J Clin Pharmacol. 1981 Aug-Sep; 21(8-9 Suppl):214S-223S.
Block RI, Farinpour R, Schlechte JA. Effects of chronic marijuana use on testosterone, luteinizing hormone, follicle stimulating hormone, prolactin and cortisol in men and women. Drug Alcohol Depend. 1991Aug; 28(2):121-8.
Block RI, Farinpour R, Braverman K. Acute effects of marijuana on cognition: relationships to chronic effects and smoking techniques. Pharmacol Biochem Behav. 1992 Nov; 43(3):907-17.
Bolla KI, Brown K, Eldreth D, Tate K, and Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology 2002 59:1337-1343.
Bolla KI, Eldreth DA, Matochik JA, and Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. NeuroImage 2005 26:480-492.
Bonnet U. Chronic cannabis abuse, delta-9-tetrahydrocannabinol and thyroid function. Pharmacopsychiatry. 2013 Jan; 46(1):35-6.
Bouaboula M, Rinaldi M, Carayon P, Carillon C, Delpech B, Shire D, Le Fur G, Casellas P. Cannabinoid-receptor expression in human leukocytes. Eur J Biochem. 1993 May 15; 214(1):173-80.
Braida D, Iosue S, Pegorini S, Sala M. Delta9-tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur J Pharmacol. 2004 Dec 3; 506(1):63-9.
Breivogel CS, Childers SR. Cannabinoid agonist signal transduction in rat brain: comparison of cannabinoid agonists in receptor binding, G-protein activation, and adenylyl cyclase inhibition. J Pharmacol Exp Ther. 2000. Oct; 295(1):328-36.
Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001 Jul; 60(1):155-63.
Brown TT, Dobs AS. Endocrine effects of marijuana. J Clin Pharmacol. 2002 Nov; 42(11 Suppl):90S-96S.
Browne RG, Weissman A. Discriminative stimulus properties of delta 9-tetrahydrocannabinol: mechanistic studies. J Clin Pharmacol. 1981 Aug-Sep; 21(8-9 Suppl):227S-234S.
Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and Start Printed Page 53708 significance of cannabis withdrawal syndrome. Am J Psychiatry. 2004 Nov; 161(11):1967-77.
Budney AJ, Hughes JR. The cannabis withdrawal syndrome. Curr Opin Psychiatry 2006 May; 19(3):233-8.
Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursae Z. Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J Subst.Abuse Treat. 2008 Dec; 35(4):362-8.
Capriotti RM, Foltin RW, Brady JV, Fischman MW. Effects of marijuana on the task-elicited physiological response. Drug Alcohol Depend. 1988 Jul; 21(3):183-7.
Cascini F, Aiello C, Di Tanna G. Increasing delta-9-tetrahydrocannabinol (Δ-9-THC) content in herbal cannabis over time: systematic review and meta-analysis. Curr Drug Abuse Rev. 2012 Mar; 5(1):32-40.
Chait LD. Subjective and behavioral effects of marijuana the morning after smoking. Psychopharmacology (Berl.) 1990; 100(3):328-33.
Chait LD, Burke KA. Preference for high- versus low-potency marijuana. Pharmacol Biochem Behav. 1994 Nov; 49(3):643-7.
Chaperon F, Soubrie P, Puech AJ, Thiebot MH. Involvement of central cannabinoid (CBI) receptors in the establishment of place conditioning in rats. Psychopharmacology (Berl). 1998 Feb; 135(4):324-32.
Cheer JF, Kendall DA, Marsden CA. Cannabinoid receptors and reward in the rat: a conditioned place preference study. Psychopharmacology (Berl). 2000 Jul; 151(1):25-30.
Cone EJ, Johnson RE, Moore JD, Roache JD. Acute effects of smoking marijuana on hormones, subjective effects and performance in male human subjects. Pharmacol Biochem Behav. 1986 Jun; 24(6): 1749-54.
Corey-Bloom J, Wolfson T, Gamst A, Jin S, Marcotte TD, Bentley H, and Gouaux B. 2012. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ 184 (10): 1143-1150.
Council on Science and Public Health Report 3. Use of cannabis for medicinal purposes. American Medical Association, Interim Meeting, Houston, Texas; November 2009.
Crawford WJ, and Merritt JC. 1979. Effects of tetrahydrocannabinol on arterial and intraocular hypertension. International journal of clinical pharmacology and biopharmacy l7(5): 191-196.
Croxford JL, Y amamura T. Cannabinoids and the immune system: potential for the treatment of inflammatory diseases? J Neuroimmunol. 2005 Sep; 166(1-2):3-18. Review.
Dalton WS, Martz R, Lemberger L, Rodda BE, Forney RB. Influence of cannabidiol on delta-9-tetrahydrocannabinol effects. Clin.Pharmacol. Ther. 1976 Mar; 19(3):300-9.
Dax EM, Pilotte NS, Adler WH, Nagel JE, Lange WR. The effects of 9-ene-tetrahydrocannabinol on hormone release and immune function. J Steroid Biochem. 1989; 34(1-6):263-70.
Degenhardt L, Hall W, Lynskey M. Testing hypotheses about the relationship between cannabis use and psychosis. Drug Alcohol Depend. 2003 Jul 20; 71(1):37-48.
Degenhardt L, Chiu WT, Conway K, Dierker L, Glantz M, Kalaydjian A, Merikangas K, Sampson N, Swendsen J, Kessler RC. Does the 'gateway' matter? Associations between the order of drug use initiation and the development of drug dependence in the National Comorbidity Study Replication. Psychol.Med 2009 Jan; 39(1):157-67.
Degenhardt L, Dierker L, Chiu WT, Medina-Mora ME, Neumark Y, Sampson N, Alonso J, Angermeyer M, Anthony JC, Bruffaerts R, et al. Evaluating the drug use "gateway" theory using cross-national data: consistency and associations of the order of initiation of drug use among participants in the WHO World Mental Health Surveys. Drug Alcohol.Depend. 2010 Apr 1; 108(1-2):84-97.
Deiana S, Fattore L, Spano MS, Cossu G, Porcu E, Fadda P, Fratta W. Strain and schedule dependent differences in the acquisition, maintenance and extinction of intravenous cannabinoid self-administration in rats. Neuropharmacology. 2007 Feb; 52(2):646-54.
Dewey, W. L., Martin, B. R., May, E. L. Cannabinoid stereoisomers: pharmacological effects. In Smith, D. F. (Ed.) CRC Handbook of stereoisomers: drugs in psychopharmacology, 317-326 (Boca Raton, FL, CRC Press),1984.
Di Marzo, V. A brief history of cannabinoid and endocannabinoid pharmacology as inspired by the work of British scientists. Trends.Pharmacol.Sci 2006 Mar; 27(3):134-40.
DEA Statistics and Facts. (n.d.) DEA Domestic Drug Seizures. http://www.justice.gov/dea/resource-center/statistics.shtml/ (accessed August 5, 2014)
Drug Enforcement Administration, Drugs of Abuse, 2005.
Drug Enforcement Administration. Sourcebook of Criminal Justice Statistics, 2003.
DSM-V. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. American Psychiatric Association. Washington, DC: American Psychiatric Publishing, 2013.
Eldridge JC, Murphy LL, Landfield PW. Cannabinoids and the hippocampal glucocorticoid receptor: recent findings and possible significance. Steroids. 1991 May; 56(5):226-31. Review.
Ellis RJ, Toperoff W, Vaida F, Van Den Brande G, Gonzales J, Gouaux B, Bentley H, and Atkinson JH. 2009. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 34(3): 672-680.
ElSohly MA, Slade D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sciences. 2005;78:539-48.
Fant RV, Heishman SJ, Bunker EB, Pickworth WB. Acute and residual effects of marijuana in humans. Pharmacol Biochem Behav. 1998 Aug; 60(4):777-84.
Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br.J Pharmacol. 2007 Nov; 152(5):795-804.
Fergusson DM, Horwood LJ, Ridder EM.Tests of causal linkages between cannabis use and psychotic symptoms. Addiction. 2005 Mar; 100(3):354-66.
Fontes MA, Bolla KI, Cunha PJ, Almeida PP, Jungerman F, Laranjeira RR; Bressan RA, Lacerda AL. Cannabis use before age 15 and subsequent executive functioning. Br.J Psychiatry 2011 Jun; 198(6):442-7.
Fried, P. A., Watkinson, B. 36- and 48-month neurobehabioral follow-up of children prenatally exposed to marijuana, cigarettes and alcohol. J. Dev. Behav. Pediatr. 1987, 8, 318-326.
Fried, P. A., Watkinson, B., Gray, R. A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marihuana, cigarettes and alcohol. Neurotoxicol. Teratol. 1992, 14, 299-311.
Fried, P. A., Watkinson, B., Gray, R. Differential effects on cognitive functioning in 9- to 12- year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol. Teratol. 1998, 20(3), 293-306.
Fried PA. Adolescents prenatally exposed to marijuana: examination of facets of complex behaviors and comparisons with the influence of in utero cigarettes. J.Clin.Pharmacol. 2002 Nov; 42(11 Suppl):97S-102S.
Fried PA, Watkinson B, Gray R. Neurocognitive consequences of marihuana—a comparison with pre-drug performance. Neurotoxicol. Teratol. 2005 Mar; 27(2):231-9.
Fung, M., Gallagher, C., Machtay, M. Lung and aeo-digestive cancers in young marijuana smokers. Tumori 1999, 85 (2), 140-142.
Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995 Aug 15; 232(1):54-61.
Gaoni, Y., Mechoulam, R. Isolation, structure, and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964, 86, 1646-1947.
Gerard, C. M., Mollereau, C., Vassart, G., Parmentier, M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991, 279, 129-34.
Ghozland S, Matthes HW, Simonin F, Filliol D, Kieffer BL, Maldonado R. Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. J Neurosci. 2002 Feb 1; 22(3):1146-54.
Gold LH, Balster RL, Barrett RL, Britt DT, Martin BR. A comparison of the Start Printed Page 53709 discriminative stimulus properties of delta 9-tetrahydrocannabinol and CP 55,940 in rats and rhesus monkeys. J Pharmacol Exp Ther. 1992 Aug; 262(2):479-86.
Goldschmidt L, Richardson GA, Willford J, Day NL. Prenatal marijuana exposure and intelligence test performance at age 6. J.Am.Acad.Child.Adolesc.Psychiatry. 2008 Mar; 47(3):254-63.
Gong H Jr, Tashkin DP, Simmons MS, Calvarese B, Shapiro BJ. Acute and subacute bronchial effects of oral cannabinoids. Clin Pharmacol Ther. 1984 Jan; 35(1):26-32.
Gong JP, Onaivi ES, lshiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: Immunohistochemical localization in rat brain. Brain Res. 2006 Feb 3; 1071(1):10-23
Gonsiorek W, Lunn C, Fan X, Narula S, Lundell D, Hipkin RW. Endocannabinoid 2- arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Mol Pharmacol. 2000 May; 57(5): 1045-50.
Gonzalez R. Acute and non-acute effects of cannabis on brain functioning and neuropsychological performance. Neuropsychol.Rev. 2007 Sep; 17(3):347-61.
Gonzalez S, Cebeira M, Fernandez-Ruiz J. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol.Biochem.Behav. 2005 Jun; 81(2):300-18.
Grant I. Foreword by Igor Grant, M.D., Director, Center for Medicinal Cannabis Research (CMCR). Neuropharmacology. 2005 Jun; 48(8): 1067.
Griffith-Lendering MF, Wigman JT, Prince van LA, Huijbregts SC, Huizink AC, Ormel J, Verhulst FC, van OJ, Swaab H, Vollebergh WA. Cannabis use and vulnerability for psychosis in early adolescence-a TRAILS study. Addiction 2012 Dec 7.
Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003; 42(4):327-60.
Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of onset of marijuana use and executive function. Psychol.Addict.Behav. 2012 Sep; 26(3):496-506.
Hall WD, Lynskey M. Is cannabis a gateway drug? Testing hypotheses about the relationship between cannabis use and the use of other illicit drugs. Drug Alcohol Rev. 2005 Jan; 24(1):39-48.
Haney M, Gunderson EW, Rabkin J, Hart CL, Vosburg SK, Comer SD, and Faltin RW. 2007. Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric.intake, mood, and sleep. Journal of acquired immune deficiency syndromes (1999) 45 (5): 545-554.
Haney M, Rabkin J, Gunderson E, and Foltin RW. 2005. Dronabinol and marijuana in HIV(+) marijuana smokers: acute effects on caloric intake and mood. Psychopharmacology 181(1): 170-178.
Haney M, Ward AS, Comer SD, Faltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology (Berl) 1999, 141(4):395-404.
Hanus, L., Breuer, A., Tchilibon, S., Shiloah, S., Goldenberg, D., Horowitz, M., Pertwee, R.G., Roos, R. A., Mechoulam, R., Pride, E. HU-308: a specific agonist for CB(2), a peripheral Cannabinoid receptor. Proc. Natl. Acad. Sci. USA 1999, 96, 14228-33.
Heishman SJ, Huestis MA, Benningfield JE, Cone EJ. Acute and residual effects of marijuana: profiles of plasma THC levels, physiological, subjective, and performance measures. Pharmacol Biochem Behav. 1990 Nov; 37(3):561-5.
Herkenham, M. Cannabinoid receptor localization in brain: Relationship to motor and reward systems. In: Kalivas, P.W., and Samson, H.H., eds. The neurobiology of drug and alcohol addiction. Ann NY Acad Sci 1992, 654, 19-32.
Herkenham, M., Lynn, A. B., Little, M. D., Johnson, M. R., Melvin, L. S., de Costa, B. R., Rice, K. C. Cannabinoid receptor localization in Brain. Proc. Natl. Acad. Sci. US A. 1990, 87, 1932-1936.
Heming, R.I.; Hooker, W.D.; and Jones, R.T. Tetrahydrocannabinol content and differences in marijuana smoking behavior. Psychopharmacology 1986, 90(2):160-162.
Hillig, K.W. Genetic evidence for speciation in Cannabis (Cannabaceae). Genetic Resources and Crop Evolution 52: 161-180, 2005.
Hirvonen, J., Goodwin, R. S., Li, C. T., Terry, G. E., Zoghbi, S. S., Morse, C., Pike, V. W., Volkow, N. D., Huestis, M.A., Innis, R. B. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol. Psychiatry. 2012(Jun), 17(6), 643-649.
Hively, R. L., Mosher, W. A., Hoffman, F. W. Isolation of trans-)9-tetrahydrocannabinol from marihuana. J. Am. Chem. Soc. 1966, 88, 1832-1833.
Hollister LE, Gillespie HK. Delta-8- and delta-9-tetrahydrocannabinol comparison in man by oral and intravenous administration. Clin.Pharmacol.Ther. 1973 May;14(3):353-7.
Hollister, L.E. Health aspects of cannabis. Pharmacological Rev. 1986, 38, 1-20.
Hollister, L.E. Cannabis. (Literature review). Acta Psychiatr Scand (Suppl) 1988, 78, 108-118.
Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Parrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004; 47 Suppl 1:345-58.
Huestis, M.A., Sampson, A.H., Holicky, B. J., Benningfield, J.E., Cone, E. J. Characterization of the absorption phase of marijuana smoking. Clin. Pharmacol. Ther. 1992a, 52, 31-41.
Huestis, M.A.; Benningfield, J.E.; and Cone, E.J. Blood Cannabinoids. 1. Absorption of THC and formation of 11-OH-THC and THC COOH during and after smoking marijuana. J Anal Toxicol 1992b, 16(5), 276-282.
Hunt CA, Jones RT. Tolerance and disposition of tetrahydrocannabinol in man. J Pharmacol Exp Ther. 1980 Oct; 215(1):35-44.
Ilan AB, Gevins A, Coleman M, Elsohly MA, de WH. Neurophysiological and subjective profile of marijuana with varying concentrations of cannabinoids. Behav.Pharmacol. 2005 Sep; 16(5-6):487-96.
Institute of Medicine. Division of Health Sciences Policy. Marijuana and Health: Report of a Study by a Committee of the Institute of Medicine, Division of Health Sciences Policy. Washington, DC: National Academy Press, 1982.
Institute of Medicine, Division of Neuroscience and Behavioral Health. Marijuana and Medicine: Assessing the Science Base. Washington DC: National Academy Press, 1999.
Johansson, E.; Halldin, M.M.; Agurell, S.; Hollister, L.E.; and Gillespie, H.K. Terminal elimination plasma half-life of delta 1-tetrahydrocannabinol (delta 1-THC) in heavy users of marijuana. Eur J Clin Pharmacol 1989, 37(3), 273-277.
Johnston, L. D., O'Malley, P. M., Miech, R. A., Bachman, J. G., & Schulenberg, J.E. (2014). Monitoring the Future national survey results on drug use: 1975-2013: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan, 84pp.
Jones, R.T.; Benowitz, N.L.; and Heming, R.I. Clinical relevance of cannabis tolerance and dependence. J Clin Pharmacol 1981, 21,143S-152S.
Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002 Nov;42(11 Suppl):58S-63S.
Justinova Z, Goldberg SR, Heishman SJ, Tanda G. Self-administration of cannabinoids by experimental animals and human marijuana smokers. Pharmacol Biochem Behav. 2005 Jun; 81(2): 285-299.
Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of delta9- tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl). 2003 Sep; 169(2): 135-40.
Justinova Z, Tanda G, Munzar P, Goldberg SR. The opioid antagonist naltrexone reduces the reinforcing effects of Delta 9 tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology.(Berl.) 2004 Apr;l 73(1-2):186-94.
Kandel, D. Stages in adolescent involvement in drug use. Science 1975; 190:912-914.
Kandel DB, Chen K. Types of marijuana users by longitudinal course. J Stud Alcohol. 2000 May; 61(3):367-78.
Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA. Cannabidiol interferes with the effects of delta 9-tetrahydrocannabinol in man. Eur.J Pharmacol. 1974 Sep;28(1):172-7.
Karniol IG, Shirakawa I, Takahashi RN, Knobel E, Musty RE. Effects of delta9- tetrahydrocannabinol and cannabinol in man. Pharmacology 1975; 13(6):502-12.
Kirk JM, de Wit H. Responses to oral delta9-tetrahydrocannabinol in frequent and Start Printed Page 53710 infrequent marijuana users. Pharmacol Biochem Behav. 1999 May; 63(1):137-42.
Kuepper R, van OJ, Lieb R, Wittchen HU, Hofler M, Henquet C. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ 2011; 342:d738.
Kurzthaler I, Hummer M, Miller C, Sperner-Unterweger B, Gunther V, Wechdorn H, Battista HJ, Fleischhacker WW. Effect of cannabis use on cognitive functions and driving ability. J Clin Psychiatry. 1999 Jun; 60(6):395-9.
Lee MH, Hancox RJ. Effects of smoking cannabis on lung function. Expert Rev.Respir.Med 2011 Aug; 5(4):537-46.
Lemberger L., Silberstein, S. D., Axelrod, J., Kopin, I. J. Marihuana: studies on the disposition and metabolism of delta-9-tetrahydrocannabinol in man. Science 1970, 170, 1320-1322.
Lemberger L., Weiss, J. L., Watanabe, A. M., Galanter, I. M., Wyatt, R. J., Cardon, P.V. Delta-9-tetrahydrocannabinol: temporal correlation of the psychological effects and blood levels after various routes of administration. New Eng. J. Med. 1972a, 286(13), 685-688.
Lemberger, L., Crabtree, R. E., Rowe, H. M. 11-Hydroxy-)9-tetrahydrocannabinol: pharmacology, disposition and metabolism of a major metabolite of marihuana in man. Science 1972b, 177, 62-63.
Lemberger L., Rubin A. The physiologic disposition of marihuana in man, Life Sci. 1975, 17, 1637-42.
Li M-C., Brady, J. E., DiMaggio, C. J., Lusardi, A. R., Tzong, K. Y., Li, G. Marijuana use and motor vehicle crashes. Epidemiologic Reviews. 2012, 34, 65-72.
Liguori A, Gatto CP, Robinson JH. Effects of marijuana on equilibrium, psychomotor performance, and simulated driving. Behav Pharmacol. 1998 Nov; 9(7):599-609.
Lile JA, Kelly TH, Hays LR. Separate and combined effects of the cannabinoid agonists nabilone and Delta(9)-THC in humans discriminating Delta(9)-THC. Drug Alcohol Depend.2011 Jul 1; 116(1-3):86-92.
Lile JA, Kelly TH, Pinsky DJ, Hays LR. Substitution profile of Delta9-tetrahydrocannabinol, triazolam, hydromorphone, and methylphenidate in humans discriminating Delta9-tetrahydrocannabinol. Psychopharmacology (Berl.) 2009 Apr; 203 (2):241-50.
Lisdahl KM, Price JS. Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. J Int Neuropsychol. Soc 2012 Jul; 18(4):678-88.
Lyons MJ, Bar JL, Panizzon MS, Toomey R, Eisen S, Xian H, Tsuang MT. Neuropsychological consequences of regular marijuana use: A twin study. Psychol Med. 2004 Oct; 34(7):1239-50.
Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995 Oct; 15(10):6552-61.
Maldonado R. Study of cannabinoid dependence in animals. Pharmacol Ther. 2002 Aug; 95(2): 153-64.
Malinowska B, Baranowska-Kuczko M, Schlicker E. Triphasic blood pressure responses to cannabinoids: Do we understand the mechanism? Br.J Pharmacol 2012 Apr; 165(7):2073-88.
Manrique-Garcia E, Zammit S, Dalman C, Hemmingsson T, Andreasson S, Allebeck P. Cannabis, schizophrenia and other non-affective psychoses: 35 years of follow-up of a population-based cohort. Psychol.Med 2012 Jun; 42(6):1321-8.
Maremmani I, Lazzeri A, Pacini M, Lovrecic M, Placidi GF, Perugi G. Diagnostic and symptomatological features in chronic psychotic patients according to cannabis use status. J Psychoactive Drugs. 2004 Jun; 36(2):235-41.
"Marijuana Scheduling Petition; Denial of Petition; Remand; Final Order," 57 Federal Register 59 (26 March 1992), pp. 10499-10508.
Martellotta MC, Cossu G, Fattore L, Gessa GL, Fratta W. Self-administration of the cannabinoid receptor agonist WIN 55,212-2 in drug-naive mice. Neuroscience. 1998 Jul; 85(2):327-30.
Matsuda, L.A., Lolait, S.J., Brownstein, M.J., Young, A.C., Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561-564.
McMahon LR. Apparent affinity estimates of rimonabant in combination with anandamide and chemical analogs of anandamide in rhesus monkeys discriminating Delta9-tetrahydrocannabinol. Psychopharmacology (Berl.) 2009 Apr; 203(2):219-28.
McMahon LR, Ginsburg BC, Lamb RJ. Cannabinoid agonists differentially substitute for the discriminative stimulus effects of Delta(9)-tetrahydrocannabinol in C57BL/6J mice. Psychopharmacology (Berl.) 2008 Jul; l98(4):487-95.
Mechoulam, R. Cannabinoid chemistry. In Mechoulam, R. (ED.) Marijuana, pp.2-88 (New York, NY, Academic Press, Inc.), 1973.
Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO. Cannabidiol—recent advances. Chem.Biodivers. 2007 Aug;4(8): 1678-92.
Mechoulam R, Shvo Y. Hashish-I: The structure of Cannabidiol. Tetrahedron. 1963; 19: 2073-78.
Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, Ross SA, Khan IA, ElSohly MA. Potency Trends of Δ9-THC and other caimabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci. 2010 Sept; 55(5): 1209-1217.
Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, McDonald K, Ward A, Poulton R, Moffitt TE. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc.Natl.Acad.Sci U.S.A 2012 Oct 2; 109(40):E2657-E2664.
Meijer JH, Dekker N, Koeter MW, Quee PJ, van Beveren NJ, Meijer CJ. Cannabis and cognitive performance in psychosis: A cross-sectional study in patients with non-affective psychotic illness and their unaffected siblings. Psychol.Med 2012 Apr; 42(4):705-16.
Mendelson JH, Mello NK. Effects of marijuana on neuroendocrine hormones in human males and females. NIDA Res Monogr. 1984; 44:97-114.
Radwan MM, Elsohly MA, Slade D, Ahmed SA, Khan IA, Ross SA. Biologically active cannabinoids from high-potency Cannabis sativa. J Nat Prod. 2009 May 22; 72(5):906-11.
Ramaekers JG, Berghaus G, van Laar M, Drummer OH. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004 Feb 7; 73(2): 109-19.
Ramaekers JG, Kauert G, van RP, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology 2006 Oct; 31(10):2296-303.
Ramaekers JG, Kauert G, Theunissen EL, Toennes SW., Moeller MR. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J.Psychopharmacol. 2009 May; 23(3):266-77.
Ramaekers JG, Moeller MR, van Ruitenbeek P, Theunissen EL, Schneider E, Kauert G. Cognition and motor control as a function of Δ9-THC concentration in serum and oral fluid: Limits of impairment. Drug and Alcohol Dependence. 2006; 85:1114-122.
"Rescheduling of the Food and Drug Administration Approved Product Containing Synthetic Dronabinol [(-)-delta9-(trans)-Tetrahydrocannabinol] in Sesame Oil and Encapsulated in Soft Gelatin Capsules From Schedule II to Schedule III; Final Rule," 64 Federal Register 127 (2 July 1999), pp.35928-35930.
Riggs PK, Vaida F, Rossi SS, Sorkin LS, Gouaux B, Grant I, Ellis RJ. A pilot study of the effects of cannabis on appetite hormones in HIV-infected adult men. Brain Res 2012 Jan 11; 1431:46-52.
Rodriguez de Fonseca F, Gorriti, M.A., Fernandez-Ruiz, J.J., Palomo, T., Ramos, J.A. Downregulation of rat brain cannabinoid binding sites after chronic delta 9-tetrahydrocannabinoil treatment. Phamacol. Biochem. Behav. 1994, 47 (1), 33-40.
Roth MD, Tashkin DP, Whittaker KM, Choi R, Baldwin GC. Tetrahydrocannabinol suppresses immune function and enhances HIV replication in the huPBL-SCID mouse. Life Sci. 2005 Aug 19; 77(14):1711-22.
Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011 Aug; 163(7):1344-64.
Sarfaraz S, Afaq F, Adhami VM, Mukhtar H. Cannabinoid receptor as a novel target for the treatment of prostate cancer. Cancer Res. 2005 Mar 1; 65(5):1635-41.
Sanudo-Peria M.C., Tsou, K., Delay, E.R., Hohman, A.G., Force, M., Walker, J.M. Start Printed Page 53711 Endogenous cannabinoids as an aversive or counter-rewarding system in the rat. Neurosci. Lett., 223, 125-128, 1997.
Mendizabal V, Zimmer A, Maldonado R. Involvement of kappa/dynorphin system in WIN 55,212-2 self-administration in mice. Neuropsychopharmacology. 2006 Sep; 31(9):1957-66.
Merritt JC, Crawford WJ, Alexander PC, Anduze AL, and Gelbart SS. 1980. Effect of marihuana on intraocular and blood pressure in glaucoma. Ophthalmology 87 (3): 222-228.
Messinis L, Kyprianidou A, Malefaki S, and Papathanasopoulos P. Neuropsychological deficits in long-term frequent cannabis users. Neurology 2006 66:737-739.
Minozzi S, Davoli M, Bargagli AM, Amato L, Vecchi S, Perucci CA. An overview of systematic reviews on cannabis and psychosis: Discussing apparently conflicting results. Drug Alcohol Rev. 2010 May; 29(3):304-17.
Mittleman MA, Lewis RA, Maclure M, Sherwood JB, and Muller JE. Triggering myocardial infarction by marijuana. Circulation. 2001; 103:2805-2809.
Nace EP, Meyers AL, Rothberg JM, Maleson F. Addicted and nonaddicted drug users. A comparison of drug usage patterns. Arch Gen Psychiatry. 1975; 32(1):77-80.
Oviedo, A., Glowa, J., Herkenham, M. Chronic cannabinoid administration alters cannabinoid receptor binding in rat brain: A quantitative autoradiographic study. Brain Res. 1993, 616, 293-302.
Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006 Sep; 58(3):389-462.
Pelayo-Teran JM, Suarez-Pinilla P, Chadi N, Crespo-Pacorro B. Gene-environment interactions underlying the effect of cannabis in first episode psychosis. Curr Pharm Des. 2012; 18(32):5024-35.
Petrocellis PL, Di Marzo V. An introduction to the endocannabinoid system: from the early to the latest concepts. Best Pract.Res.Clin.Endocrinol.Metab. 2009 Feb; 23(1):1-15.
Piomelli D. The endocannabinoid system: A drug discovery perspective. Curr Opin Investig Drugs. 2005 Jul; 6(7):672-9.
Pletcher MJ, Vittinghoff E, Kalhan R, Richman J, Safford M, Sidney S, Lin F, Kertesz S. Association between marijuana exposure and pulmonary function over 20 years. JAMA 2012 Jan 11; 307(2):173-81.
Pollastro F, Taglialatela-Scafati O, Allara M, Munoz E, Di Marzo V, De Petrocellis L, Appendino G. Bioactive prenylogous cannabinoid from fiber hemp (Cannabis sativa). J Nat Prod. 2011 Sep 23;74(9):2019-22.
Pope HG Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Cognitive measures in long term cannabis users. J Clin Pharmacol. 2002 Nov; 42(11 Suppl):41S-47S. Review.
Scherrer JF, Grant JD, Duncan AE, Sartor CE, Haber JR, Jacob T, Bucholz KK. Subjective effects to cannabis are associated with use, abuse and dependence after adjusting for genetic and environmental influences. Drug Alcohol Depend. 2009 Nov 1; 105(1-2):76-82.
Schiffman J, Nakamura B, Earleywine M, LaBrie J. Symptoms of schizotypy precede cannabis use. Psychiatry Res. 2005 Mar 30; 134(1):37-42.
Schreiner AM, Dunn ME. Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: A meta-analysis. Exp.Clin Psychopharmacol. 2012 Oct; 20(5):420-9.
Sidney S. Cardiovascular consequences of marijuana use. J Clin Pharmacol. 2002 Nov; 42(11 Suppl):64S-70S.
Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J; Marijuana Treatment Project Research Group. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002 Mar 6; 287(9): 1123-31.
Stirling J, Lewis S, Hopkins R, White C. Cannabis use prior to first onset psychosis predicts spared neurocognition at 10-year follow-up. Schizophr Res. 2005 Jun 1; 75(1):135-7.
Substance Abuse and Mental Health Services Administration, Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits. HHS Publication No. (SMA) 13-4760, DAWN Series D-39. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013.
Substance Abuse and Mental Health Services Administration, Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013.
Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Treatment Episode Data Set (FEDS): 2001-2011. National Admissions to Substance Abuse Treatment Services. BHSIS Series S-65, HHS Publication No. (SMA) 13-4772. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013.
Tait RJ, Mackinnon A, Christensen H. Cannabis use and cognitive function: 8-year trajectory in a young adult cohort. Addiction 2011 Dec; 106(12):2195-203.
Tanasescu R, Constantinescu CS. Cannabinoids and the immune system: an overview. Immunobiology. 2010 Aug; 215(8):588-97.
Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000 Nov; 3(11):1073-4.
Tashkin DP. Smoked marijuana as a cause of lung injury. Monaldi Arch Chest Dis. 2005 Jun; 63(2):93-100.
Tashkin DP, Shapiro BJ, and Frank IM. 1974. Acute effects of smoked marijuana and oral delta9-tetrahydrocannabinol on specific airway conductance in asthmatic subjects. The American review of respiratory disease 109 (4): 420-428.
Tashkin, DP, Zhang, ZF, Greenland, S, Cozen, W, Mack, TM, Morgenstern, H. Marijuana Use and Lung Cancer: Results of a Case-Control Study. Abstract #A 777, American Thoracic Society meeting, May 24, 2006.
The Plant List (2010). Version 1. Published on the Internet; http://www.theplantlist.org/ (accessed September 20, 2013)
Theunissen EL, Kauert GF, Toennes SW., Moeller MR, Sambeth A, Blanchard MM, Ramaekers JG. Neurophysiological functioning of occasional and heavy cannabis users during THC intoxication. Psychopharmacology (Berl.) 2012 Mar;220(2):341-50.
Trabert B, Sigurdson AJ, Sweeney AM, Strom SS, McGlynn KA. Marijuana use and testicular germ cell tumors. Cancer. 2011 Feb 15; 117: 848-853.
Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol. 1997 Jul; 78(1):43-50.
van der Meer FJ, Velthorst E, Meijer CJ, Machielsen MW, de HL. Cannabis use in patients at clinical high risk of psychosis: Impact on prodromal symptoms and transition to psychosis. Curr Pharm Des. 2012; 18(32):5036-44.
van Gastel WA, Wigman JT, Monshouwer K, Kahn RS, van OJ, Boks MP, Vollebergh WA. Cannabis use and subclinical positive psychotic experiences in early adolescence: Findings from a Dutch survey. Addiction 2012 Feb; 107(2):381-7.
Van Gundy K, Rebellon CJ. A Life-course Perspective on the "Gateway Hypothesis." J Health Soc Behav. 2010 Sep; 51(3):244-59.
van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H. Cannabis use and psychosis: A longitudinal population-based study. Am J Epidemiol. 2002 Aug 15; 156(4):319-27.
Vandrey RG, Budney AJ, Moore BA, Hughes JR. A cross-study comparison of cannabis and tobacco withdrawal. Am J Addict. 2005 Jan-Feb; 14(1):54-63.
Vandrey RG, Budney AJ, Hughes JR, Liguori A. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008 Jan 1; 92(1-3):48-54.
Vann RE, Gamage TF, Warner JA, Marshall EM, Taylor NL, Martin BR, Wiley JL. Divergent effects of cannabidiol on the discriminative stimulus and place conditioning effects of Delta(9) tetrahydrocannabinol. Drug Alcohol.Depend. 2008 Apr 1; 94(1-3):191-8.
Vanyukov MM, Tarter RE, Kirillova GP, Kirisci L, Reynolds MD, Kreek MJ, Conway KP, Maher BS, Iacono WG, Bierut L, Neale MC, Clark DB, Ridenour TA. Common liability to addiction and "gateway hypothesis": Theoretical, empirical and evolutionary perspective. DrugAlcohol Depend. 2012 Jun; 123 Suppl 1: S3-17.
von Sydow K, Lieb R, Pfister H, Hofler M, Wittchen HU.What predicts incident use Start Printed Page 53712 of cannabis and progression to abuse and dependence? A 4-year prospective examination of risk factors in a community sample of adolescents and young adults. Drug Alcohol Depend. 2002 Sep 1; 68(1):49-64.
Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H. Comparison of the subjective effects of Delta (9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl). 2002 Jun; 161(4):331-9.
Wagner JA, Varga K, Kunos G. Cardiovascular actions of cannabinoids and their generation during shock. J Mol Med. 1998 Nov-Dec; 76(12):824-36.
Ware MA, Wang T, Shapiro S, Robinson A, Ducruet T, Huynh T, Gamsa A, Bennett GJ, and Collet JP. 2010. Smoked cannabis for chronic neuropathic pain: A randomized controlled trial. CMAJ: Canadian Medical Association journal = journal de l'Association medicate canadienne 182(14): E694-E701.
Wesson DR, Washburn P. Current patterns of drug abuse that involve smoking. NIDA. Res. Monogr. 1990; 99: 5-11.
Wiley JL, Barrett RL, Britt DT, Balster RL, Martin BR. Discriminative stimulus effects of delta 9-tetrahydrocannabinol and delta 9-11-tetrahydrocannabinol in rats and rhesus monkeys. Neuropharmacology. 1993 Apr; 32(4):359-65.
Wiley JL, Huffman JW, Balster RL, Martin BR. Pharmacological specificity of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rhesus monkeys. Drug Alcohol Depend. 1995 Nov; 40(1):81-6.
Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, and Donaghe H. 2013. Low-Dose Vaporized Cannabis Significantly Improves Neuropathic Pain. The journal of pain: Official journal of the American Pain Society.
Wilsey B, Marcotte T, Tsodikov A, Millman J, Bentley H, Gouaux B, and Fishman S. 2008. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. The journal of pain: Official journal of the American Pain Society 9(6): 506-521.
Wu X, French ED. Effects of chronic delta9-tetrahydrocannabinol on rat midbrain dopamine neurons: an electrophysiological assessment. Neuropharmacology. 2000 Jan 28; 39(3):391-8.
Yucel M, Bora E, Lubman DI, Solowij N, Brewer WJ, Cotton SM, Conus P, Takagi MJ, Fomito A, Wood SJ, et al. The impact of cannabis use on cognitive functioning in patients with schizophrenia: a meta-analysis of existing findings and new data in a first-episode sample. Schizophr.Bull. 2012 Mar; 38(2):316-30.
Zeiger JS, Haberstick BC, Corley RP, Ehringer MA, Crowley TJ, Hewitt JK, Hopfer CJ,
Stallings MC, Young SE., Rhee SH. Subjective effects to marijuana associated with marijuana use in community and clinical subjects. Drug Alcohol Depend. 2010 Jun 1; 109(1-3):161-6.
Zhang ZF, Morgenstern H, Spitz MR, Tashkin DP, Yu GP, Marshall JR, Hsu TC, Schantz SP. Marijuana use and increased risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol Biomarkers Prev. 1999 Dec;8(12): 1071-8.
Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology (Berl.) 1982; 76(3):245-50.
The Medical Application of Marijuana: A Review of Published Clinical Studies
March 19, 2015
Prepared by:
U.S. Food and Drug Administration
Center for Drug Evaluation and Research (FDA/CDER)
Controlled Substance Staff (CSS)
| 1. Introduction | 71 |
| 2. Methods | 73 |
| 2.1 Define the Objective of the Review | 73 |
| 2.2 Define "Marijuana" | 73 |
| 2.3 Define "Adequate and Well-Controlled Clinical Studies" | 74 |
| 2.4 Search Medical Literature Databases and Identify Relevant Studies | 74 |
| 2.5 Review and Analyze Qualifying Clinical Studies | 77 |
| 3. Results and Discussion | 77 |
| 3.1 Neuropathic Pain | 77 |
| 3.1.1 Neuropathic Pain Associated with HIV-Sensory Neuropathy | 77 |
| 3.1.2 Central and Peripheral Neuropathic Pain | 81 |
| 3.2 Appetite Stimulation in HIV | 85 |
| 3.3 Spasticity in Multiple Sclerosis | 88 |
| 3.4 Asthma | 89 |
| 3.5 Glaucoma | 91 |
| 3.6 Conclusions | 91 |
| 3.6.1 Conclusions for Chronic Neuropathic Pain | 91 |
| 3.6.2 Conclusions for Appetite Stimulation in HIV | 92 |
| 3.6.3 Conclusions for Spasticity in MS | 92 |
| 3.6.4 Conclusions for Asthma | 92 |
| 3.6.5 Conclusions for Glaucoma | 93 |
| 3.7 Design Challenges for Future Studies | 93 |
| 3.7.1 Sample Size | 93 |
| 3.7.2 Marijuana Dose Standardization | 94 |
| 3.7.3 Acute vs. Chronic Therapeutic Marijuana Use | 95 |
| 3.7.4 Smoking as a Route of Administration | 96 |
| 3.7.5 Difficulty in Blinding of Drug Conditions | 96 |
| 3.7.6 Prior Marijuana Experience | 97 |
| 3.7.7 Inclusion and Exclusion Criteria | 98 |
| 3.7.8 Number of Female Subjects | 98 |
| Appendix (Tables) | 103 |
| List of Figure: | |
| Figure 1: Identification of Studies from PubMed Search | 76 |
| List of Tables: | |
| Table 1: Randomized, controlled, double-blind trials examining smoked marijuana in treatment of neuropathic pain | 103 |
| Table 2: Randomized, controlled, double-blind trials examining smoked marijuana in treatment of appetite stimulation in HIV/AIDS | 108 |
| Table 3: Randomized, controlled, double-blind trails examining smoked marijuana in treatment of spasticity in Multiple Sclerosis | 111 |
| Table 4: Randomized, controlled, double-blind trails examining smoked marijuana in treatment of intraocular pressure in Glaucoma | 112 |
| Table 5: Randomized, controlled, double-blind trails examining smoked marijuana in treatment of asthma | 114 |
Start Printed Page 53713
Executive Summary
Marijuana is a Schedule I substance under the Controlled Substances Act (CSA). Schedule I indicates a high potential for abuse, no currently accepted medical use in the United States, and a lack of accepted safety for use under medical supervision. To date, marijuana has not been subject to an approved new drug application (NDA) that demonstrates its safety and efficacy for a specific indication under the Food Drug and Cosmetic Act (FDCA).
Nevertheless, as of October 2014, twenty-three states and the District of Columbia have passed state-level medical marijuana laws that allow for marijuana use within that state; similar bills are pending in other states.
The present review was undertaken by the Food and Drug Administration (FDA) to analyze the clinical studies published in the medical literature investigating the use of marijuana in any therapeutic areas. First, we discuss the context for this scientific review. Next, we describe the methods used in this review to identify adequate and well-controlled studies evaluating the safety and efficacy of marijuana for particular therapeutic uses.
The FDA conducted a systematic search for published studies in the medical literature that meet the described criteria for study design and outcome measures prior to February 2013. While not part of our systematic review, we have continued to routinely follow the literature beyond that date for subsequent studies. Studies were considered to be relevant to this review if the investigators administered marijuana to patients with a diagnosed medical condition in a well-controlled, double-blind, placebo-controlled clinical trial. Of the eleven studies that met the criteria for review, five different therapeutic areas were investigated:
- Five studies examined chronic neuropathic pain
- Two studies examined appetite stimulation in human immunodeficiency virus (HIV) patients
- Two studies examined glaucoma
- One study examined spasticity and pain in multiple sclerosis (MS)
- One study examined asthma.
For each of these eleven clinical studies, information is provided regarding the subjects studied, the drug conditions tested (including dose and method of administration), other drugs used by subjects during the study, the physiological and subjective measures collected, the outcome of these measures comparing treatment with marijuana to placebo, and the reported and observed adverse events. The conclusions drawn by the investigators are then described, along with potential limitations of these conclusions based on the study design. A brief summary of each study's findings and limitations is provided at the end of the section.
The eleven clinical studies that met the criteria and were evaluated in this review showed positive signals that marijuana may produce a desirable therapeutic outcome, under the specific experimental conditions tested. Notably, it is beyond the scope of this review to determine whether these data demonstrate that marijuana has a currently accepted medical use in the United States. However, this review concludes that these eleven clinical studies serve as proof-of-concept studies, based on the limitations of their study designs, as described in the study summaries. Proof-of-concept studies provide preliminary evidence on a proposed hypothesis regarding a drug's effect. For drugs under development, the effect often relates to a short-term clinical outcome being investigated. Proof-of-concept studies serve as the link between preclinical studies and dose ranging clinical studies. Therefore, proof-of-concept studies are not sufficient to demonstrate efficacy of a drug because they provide only preliminary information about the effects of a drug. However, the studies reviewed produced positive results, suggesting marijuana should be further evaluated as an adjunct treatment for neuropathic pain, appetite stimulation in HIV patients, and spasticity in MS patients.
The main limitations identified in the eleven studies testing the medical applications of marijuana are listed below:
- The small numbers of subjects enrolled in the studies, which limits the statistical analyses of safety and efficacy.
- The evaluation of marijuana only after acute administration in the studies, which limits the ability to determine efficacy following chronic administration.
- The administration of marijuana typically through smoking, which exposes ill patients to combusted material and introduces problems with determining the doses delivered.
- The potential for subjects to identify whether they received marijuana or placebo, which breaks the blind of the studies.
- The small number of cannabinoid naïve subjects, which limits the ability to determine safety and tolerability in these subjects.
- The low number of female subjects, which makes it difficult to generalize the study findings to subjects of both genders.
Thus, this review discusses the following methodological changes that may be made in order to resolve these limitations and improve the design of future studies which examine the safety and efficacy of marijuana for specific therapeutic indications:
- Determine the appropriate number of subjects studied based on recommendations in various FDA Guidances for Industry regarding the conduct of clinical trials for specific medical indications.
- Administer consistent and reproducible doses of marijuana based on recommendations in the FDA Guidance for Industry: Botanical Drug Products (2004). [27]
- Evaluate the effects of marijuana under therapeutic conditions following both acute and chronic administration.
- Consider alternatives to smoked marijuana (e.g., vaporization).
- Address and improve whenever possible the difficulty in blinding of marijuana and placebo treatments in clinical studies.
- Evaluate the effect of prior experience with marijuana with regard to the safety and tolerability of marijuana.
- Strive for gender balance in the subjects used in studies.
In conclusion, the eleven clinical studies conducted to date do not meet the criteria required by the FDA to determine if marijuana is safe and effective in specific therapeutic areas. However, the studies can serve as proof-of-concept studies and support further research into the use of marijuana in these therapeutic indications. Additionally, the clinical outcome data and adverse event profiles reported in these published studies can beneficially inform how future research in this area is conducted. Finally, application of the recommendations listed above by investigators when designing future studies could greatly improve the available clinical data that can be used to determine if marijuana has validated and reliable medical applications.
1. Introduction
In response to citizen petitions submitted to the Drug Enforcement Administration (DEA) requesting DEA to reschedule marijuana, the DEA Administrator requested that the U.S. Department of Health and Human Start Printed Page 53714 Services (HHS) provide a scientific and medical evaluation of the available information and a scheduling recommendation for marijuana, in accordance with 21 U.S.C. 811(b). The Secretary of HHS is required to consider in a scientific and medical evaluation eight factors determinative of control under the Controlled Substance Act (CSA). Administrative responsibilities for evaluating a substance for control under the CSA are performed by the Food and Drug Administration (FDA), with the concurrence of the National Institute on Drug Abuse (NIDA). Part of this evaluation includes an assessment of whether marijuana has a currently accepted medical use in the United States. This assessment necessitated a review of the available data from published clinical studies to determine whether there is adequate scientific evidence of marijuana's effectiveness.
Under Section 202 of the CSA, marijuana is currently controlled as a Schedule I substance (21 U.S.C. 812). Schedule I includes those substances that have a high potential for abuse, have no currently accepted medical use in treatment in the United States, and lack accepted safety for use under medical supervision (21 U.S.C. § 812(b)(1)(A)-(C)).
A drug product which has been approved by FDA for marketing in the United States is considered to have a "currently accepted medical use." Marijuana is not an FDA-approved drug product, as a New Drug Application (NDA) or Biologics License application (BLA) for marijuana has not been approved by FDA. However, FDA approval of an NDA is not the only means through which a drug can have a currently accepted medical use in the United States.
In general, a drug may have a "currently accepted medical use" in the United States if the drug meets a five-part test. Established case law (Alliance for Cannabis Therapeutics v. DEA, 15 F.3d 1131, 1135 (D.C. Cir. 1994)) upheld the Administrator of DEA's application of the five-part test to determine whether a drug has a "currently accepted medical use." The following describes the five elements that characterize "currently accepted medical use" for a drug:[28]
i. the drug's chemistry must be known and reproducible.
"The substance's chemistry must be scientifically established to permit it to be reproduced into dosages which can be standardized. The listing of the substance in a current edition of one of the official compendia, as defined by section 201(j) of the Food, Drug and Cosmetic Act, 21 U.S.C. 321(j), is sufficient to meet this requirement."
ii. there must be adequate safety studies.
"There must be adequate pharmacological and toxicological studies, done by all methods reasonably applicable, on the basis of which it could fairly and responsibly be concluded, by experts qualified by scientific training and experience to evaluate the safety and effectiveness of drugs, that the substance is safe for treating a specific, recognized disorder."
iii. there must be adequate and well-controlled studies proving efficacy.
"There must be adequate, well-controlled, well-designed, well-conducted, and well-documented studies, including clinical investigations, by experts qualified by scientific training and experience to evaluate the safety and effectiveness of drugs, on the basis of which it could be fairly and responsibly concluded by such experts that the substance will have the intended effect in treating a specific, recognized disorder."
iv. the drug must be accepted by qualified experts.
"The drug has a New Drug Application (NDA) approved by the Food and Drug Administration, pursuant to the Food, Drug and Cosmetic Act, 21 U.S.C. 355. Or, a consensus of the national community of experts, qualified by scientific training and experience to evaluate the safety and effectiveness of drugs, accepts the safety and effectiveness of the substance for use in treating a specific, recognized disorder. A material conflict of opinion among experts precludes a finding of consensus." and
v. the scientific evidence must be widely available.
"In the absence of NDA approval, information concerning the chemistry, pharmacology, toxicology, and effectiveness of the substance must be reported, published, or otherwise widely available, in sufficient detail to permit experts, qualified by scientific training and experience to evaluate the safety and effectiveness of drugs, to fairly and responsibly conclude the substance is safe and effective for use in treating a specific, recognized disorder."
One way to pass the five-part test for having "currently accepted medical use" is through submission of an NDA or BLA which is approved by FDA. However, FDA approval of an NDA or BLA is not required for a drug to pass the five-part test.
This review focuses on FDA's analysis of one element of the five-part test for determining whether a drug has "currently accepted medical use". Specifically, the present review assesses the 3rd criterion that addresses whether marijuana has "adequate and well-controlled studies proving efficacy". Thus, this review evaluates published clinical studies that have been conducted using marijuana in subjects who have a variety of medical conditions by assessing the adequacy of the summarized study designs and the study data. The methodology for selecting the studies that were evaluated is delineated below.
FDA's evaluation and conclusions regarding the remaining four criteria for whether marijuana has a "currently accepted medical use," as well as the eight factors pertaining to the scheduling of marijuana, are outside the scope of this review. A detailed discussion of these factors is contained in FDA's scientific and medical evaluation of marijuana.
2. Methods
The methods for selecting the studies to include in this review involved the following steps, which are described in detail in the subsections below:
1. Define the objective of the review.
2. Define "marijuana" in order to facilitate the medical literature search for studies that administered the substance,
3. Define "adequate and well-controlled studies" in order to facilitate the search for relevant data and literature,
4. Search medical literature databases and identify relevant adequate and well-controlled studies, and
5. Review and analyze the adequate and well-controlled clinical studies to determine if they demonstrate efficacy of marijuana for any therapeutic indication.
2.1 Define the Objective of the Review
The objective of this review is to assess the study designs and resulting data from clinical studies published in the medical literature that were conducted with marijuana (as defined below) as a treatment for any therapeutic indication, in order to determine if they meet the criteria of "adequate and well-controlled studies proving efficacy".
2.2 Define "Marijuana"
In this review, the term "marijuana" refers to the flowering tops or leaves of the Cannabis plant. There were no restrictions on the route of administration used for marijuana in the studies.
Studies which administered individual cannabinoids (whether Start Printed Page 53715 experimental substances or marketed drug products) or marijuana extracts were excluded from this review. Additionally, studies of administered neutral plant material or placebo marijuana (marijuana with all cannabinoids extracted) that had subsequently been supplemented by the addition of specific amounts of THC or other cannabinoids were also excluded (Chang et al., 1979).
2.3 Define "Adequate and Well-Controlled Clinical Studies"
The criteria for an "adequate and well-controlled study" for purposes of determining the safety and efficacy of a human drug is defined under the Code of Federal Regulations (CFR) in 21 CFR 314.126. The elements of an adequate and well-controlled study as described in 21 CFR 314.126 can be summarized as follows:
1. The main objective must be to assess a therapeutically relevant outcome.
2. The study must be placebo-controlled.
3. The subjects must qualify as having the medical condition being studied.
4. The study design permits a valid comparison with an appropriate control condition.
5. The assignment of subjects to treatment and control groups must be randomized.
6. There is minimization of bias through the use of a double-blind study design.
7. The study report contains a full protocol and primary data.
8. Analysis of the study data is appropriately conducted.
As noted above, the current review examines only those data available in the public domain and thus relies on clinical studies published in the medical literature. Published studies by their nature are summaries that do not include the level of detail required by studies submitted to FDA in an NDA.
While the majority of the elements defining an adequate and well-controlled study can be satisfied through a published paper (elements #1-6), there are two elements that cannot be met by a study published in the medical literature: element #7 (availability of a study report with full protocol and primary data) and element #8 (a determination of whether the data analysis was appropriate). Thus, for purposes of this review, only elements #1-6 will be used to qualify a study as being adequate and well-controlled.
2.4 Search Medical Literature Databases and Identify Relevant Studies
We identified randomized, double-blind, placebo-controlled clinical studies conducted with marijuana to assess marijuana's efficacy in any therapeutic indication. Two primary medical literature databases were searched for all studies posted to the databases prior to February 2013:[29]
- PubMed: PubMed is a database of published medical and scientific studies that is maintained by the U.S. National Library of Medicine (NLM) at NIH as a part of the Entrez system of information retrieval. PubMed comprises more than 24 million citations for biomedical literature from MEDLINE, life science journals, and online books (http://www.ncbi.nlm.nih.gov/pubmed).
- ClinicalTrials.gov: ClinicalTrials.gov is a database of publicly and privately supported clinical studies that is maintained by the NLM. Information about the clinical studies is provided by the Sponsor or Principal Investigator of the study. Information about the studies is submitted to the Web site ("registered") when the studies begin, and is updated throughout the study. In some cases, results of the study or resulting publication citations are submitted to the Web site after the study ends (https://clinicaltrials.gov/ct2/about-site/background).
ClinicalTrials.gov was searched for all studies administering marijuana. The results of this search were used to confirm that no completed studies with published data were missed in the literature search. During the literature search, references found in relevant studies and systematic reviews were evaluated for additional relevant citations. All languages were included in the search. The PubMed search yielded a total of 566 abstracts.[30] Of these abstracts, a full-text review was conducted with 85 papers to assess eligibility. From this evaluation, only eleven of 85 studies met the 6 CFR elements for inclusion as adequate and well-controlled studies.
Figure 1 (below) provides an overview of the process used to identify studies from the PubMed search. The eleven studies reviewed were published between 1974 and 2013. Ten of these studies were conducted in the United States and one study was conducted in Canada. These eleven studies examined the effects of smoked and vaporized marijuana for the indications of chronic neuropathic pain, spasticity related to multiple sclerosis (MS), appetite stimulation in patients with human immunodeficiency virus (HIV), glaucoma, and asthma. All included studies used adult patients as subjects. All studies conducted in the United States were conducted under an IND as Phase 2 investigations.
Start Printed Page 53716

Two qualifying studies, which assessed marijuana for glaucoma, were previously reviewed in the 1999 Institute of Medicine (IOM) report entitled "Marijuana and Medicine: Assessing the Science Base".[31] We did our own analysis of these two studies and concurred with the conclusions in the IOM report. Thus, a detailed discussion of the two glaucoma studies is not included in the present review. The present review only discusses 9 of the identified 11 studies. For a summary of the study design for all eleven qualifying studies, see Tables 1-5 (located in the Appendix).
Based on the selection criteria for relevant studies described in Section 2.3 (Define Adequate and Well-Controlled Clinical Studies), a number of clinical studies that investigated marijuana, as defined in this review, were excluded from this review. Studies that examined the effects of marijuana in healthy subjects were excluded because they did not test a patient population with a medical condition (Flom et al., 1975; Foltin et al., 1986; Foltin et al., 1988; Hill et al., 1974; Milstein et al., 1974; Milstein et al., 1975; Soderpalm et al., 2001; Wallace et al., 2007; Greenwald and Stitzer, 2000). A 1975 study by Tashkin et al. was excluded because it had a single-blind, rather than double-blind, study design. Two other studies were excluded because the primary outcome measure assessed safety rather than a therapeutic outcome (Greenberg et al., 1994; Abrams et al., 2003).
2.5 Review and Analyze Qualifying Clinical Studies
Qualified clinical studies that evaluated marijuana for therapeutic purposes were examined in terms of adequacy of study design including method of drug administration, study size, and subject inclusion and exclusion criteria. Additionally, the measures and methods of analysis used in the studies to assess the treatment effect were examined.
3. Results and Discussion
The eleven qualifying studies in this review assessed a variety of therapeutic indications. In order to better facilitate analysis and discussion of the studies, the following sections group the studies by therapeutic area. Within each section, each individual study is summarized in terms of its design, outcome data and important limitations. This information is also provided in the Appendix in tabular form for each study.
3.1 Neuropathic Pain
Five randomized, double-blind, placebo-controlled Phase 2 clinical studies have been conducted to examine the effects of inhaled marijuana smoke on neuropathic pain associated with HIV-sensory neuropathy (Abrams et al., 2007; Ellis et al., 2009) and chronic neuropathic pain from multiple causes (Wilsey et al., 2008; Ware et al., 2010; Wilsey et al., 2013). Table 1 of the Appendix summarizes these studies. Start Printed Page 53717
3.1.1 Neuropathic Pain Associated with HIV-Sensory Neuropathy
Two studies examined the effect of marijuana to reduce the pain induced by HIV-sensory neuropathy.
Abrams et al. (2007) conducted the first study entitled, "Cannabis in painful HIV-associated sensory neuropathy: A randomized placebo-controlled trial". The subjects were 50 adult patients with uncontrolled HIV-associated sensory neuropathy, who had at least 6 experiences with smoking marijuana. The subjects were split into two parallel groups of 25 subjects each. More than 68% of subjects were current marijuana users, but all individuals were required to discontinue using marijuana prior to the study. Most subjects were taking medication for pain during the study, with the most common medications being opioids and gabapentin. Upon entry into the study, subjects had an average daily pain score of at least 30 on a 0-100 visual analog scale (VAS).
Subjects were randomized to receive either smoked marijuana (3.56% THC[32] ) or smoked placebo cigarettes three times per day for 5 days, using a standardized cued smoking procedure: (1) 5 second inhale, (2) 10 second holding smoke in the lungs, (3) 40 second exhale and breathing normally between puffs. The authors did not specify how many puffs the subjects smoked at each smoking session, but they stated that one cigarette was smoked per smoking session.
Primary outcome measures included daily VAS ratings of chronic pain and the percentage of subjects who reported a result of more than 30% reduction in pain intensity. The ability of smoked marijuana to induce acute analgesia was assessed using both thermal heat model and capsaicin sensitization model, while anti-hyperalgesia was assessed with brush and von Frey hair stimuli. The immediate analgesic effects of smoked marijuana was assessed using a 0-100 point VAS at 40-minute intervals three times before and three times after the first and last smoking sessions, which was done to correspond to the time of peak plasma cannabinoid levels. Notably, not all subjects completed the induced pain portion of the study (n = 11 in marijuana group, 9 in placebo group) because of their inability to tolerate the stimuli. Throughout the study, subjects also completed the Profile of Mood States (POMS) questionnaire, as well as subjective VAS measures of anxiety, sedation, disorientation, paranoia, confusion, dizziness, and nausea.
As a result, the median daily pain was reduced 34% by smoked marijuana compared to 17% by placebo (p = 0.03). Fifty-two percent of subjects who smoked marijuana reported a >30% reduction in pain compared to 24% in the placebo group (p = 0.04). Although marijuana reduced experimentally-induced hyperalgesia (p ≤ 0.05) during the first smoking sessions, marijuana did not alter responses to acutely painful stimuli.
There were no serious AEs and no episodes of hypertension, hypotension, or tachycardia requiring medical intervention. No subjects withdrew from the study for drug related reasons. Subjects in the marijuana group reported higher ratings on the subjective measures of anxiety, sedation, disorientation, confusion, and dizziness compared to the placebo group. There was one case of severe dizziness in a marijuana-treated subject. By the end of the study, subjects treated with marijuana and placebo reported a reduction in total mood disturbance as measured by POMS.
The authors conclude that smoked marijuana effectively reduced chronic neuropathic pain from HIV-associated sensory neuropathy with tolerable side effects. However, limitations of this study include: maintenance of subjects on other analgesic medication while being tested with marijuana and a lack of information about the number of puffs during each inhalation of smoke. These limitations make it difficult to conclude that marijuana has analgesic properties on its own and that the actual AEs experienced during the study in response to marijuana are tolerable. However, the study produced positive results suggesting that marijuana should be studied further as an adjunct treatment for uncontrolled HIV-associated sensory neuropathy.
Ellis et al. (2009) conducted a more recent study entitled "Smoked medicinal cannabis for neuropathic pain in HIV: A randomized, crossover clinical trial". The subjects were 28 HIV-positive adult male patients with intractable neuropathic pain that was refractory to the effects of at least two drugs taken for analgesic purposes. Upon entry into the study, subjects had a mean score of >5 on the Pain Intensity subscale of the Descriptor Differential Scale (DDS). Subjects were allowed to continue taking their current routine of pain medications, which included opioids, non-narcotic analgesics, antidepressants, and anticonvulsants. Previous experience with marijuana was not required for participation in the study, but 27 of 28 subjects (96%) reported previous experience with marijuana. However, of these 27 experienced subjects, 63% (n = 18) reported no marijuana use within the past year.
The study procedures compared the effects of the target dose of marijuana and placebo during two treatment periods lasting 5 days, with 2 weeks washout periods. The marijuana strengths available were 1%, 2%, 4%, 6%, or 8% THC concentration by weight. Subjects smoked marijuana or placebo cigarettes four times per day, approximately 90-120 minutes apart, using a standardized cued smoking procedure: (1) 5 second smoke inhalation, (2) 10 second hold of smoke in lungs, (3) 40 second exhale and normal breathing between puffs. The investigators did not provide a description of the number of puffs taken at any smoking session. All subjects practiced the smoking procedures using placebo marijuana prior to test sessions.
On the first day of each test period, dose titration occurred throughout the four smoking sessions scheduled for that day, with a starting strength of 4% THC concentration. Subjects were allowed to titrate to a personalized "target dose", which was defined as the dose that provided the best pain relief without intolerable adverse effects. This dose titration was accomplished by allowing subjects to either increase the dose incrementally (to 6% or 8% THC) to improve analgesia, or to decrease the dose incrementally (to 1% or 2% THC) if AEs were intolerable. For the next 4 days of each test period, the subjects smoked their target dose during each of the four daily smoking sessions. To maintain the blind, placebo marijuana was represented as containing 1%-8% THC, even though it did not contain any cannabinoids.
The primary outcome measure was the change in pain magnitude on the DDS at the end of each test period compared to baseline, with a clinically significant level of analgesia considered to be a reduction in pain of at least 30%. Additional measures included the POMS, the Sickness Impact Profile (SIP), the Brief Symptom Inventory (BSI) and the UKU Side Effect Rating Scale and a subjective highness/sedation VAS.
During the marijuana treatment week, 19 subjects titrated to the 2%-4% THC dose while the 6%-8% dose was preferred by 8 subjects and 1 subject chose the 1% dose. In contrast, during the placebo treatment week, all 28 subjects titrated to the highest possible Start Printed Page 53718 dose of "8% THC" that contained no actual cannabinoids, suggesting that placebo treatment provided little analgesic relief.
The degree of pain reduction was significantly greater after administration of marijuana compared to placebo (median change of 3.3 points on DDS, p = 0.016). The median change from baseline in VAS pain scores was −17 for marijuana treatment compared to −4 for placebo treatment (p < 0.001). A larger proportion of subjects who were treated with marijuana (0.46) reported a >30% reduction in pain, compared to placebo (0.18). Additionally, the authors report improvements in total mood disturbance, physical disability, and quality of life as measured on POMS, SIP, and BSI scales after both placebo and marijuana treatment (data not provided in paper).
In terms of safety, there were no alterations in HIV disease parameters in response to marijuana or placebo. The authors report that marijuana led to a greater degree of UKU responses as well as AEs such as difficulty in concentration, fatigue, sleepiness or sedation, increased duration of sleep, reduced salivation and thirst compared to placebo (data not provided in paper). Two subjects withdrew from the study because of marijuana-related AEs: one subject developed an intractable smoking-related cough during marijuana administration and the sole marijuana-naïve subject in the study experienced an incident of acute cannabis-induced psychosis.[33]
The authors conclude that smoked marijuana effectively reduced chronic neuropathic pain from HIV-associated sensory neuropathy. The limitations of this study include: a lack of information about the number of puffs during each inhalation of smoke; a lack of information about the specific timing of the subjective assessments and collection of AEs relative to initiation of the smoking sessions; and the inclusion of only one marijuana-naïve subject. These limitations make it difficult to conclude that the actual AEs experienced during the study in response to marijuana are tolerable. It is especially concerning that the only marijuana-naïve subject left the study because of serious psychiatric responses to marijuana exposure at analgesic doses. However, the study produced positive results suggesting that marijuana should be studied further as an adjunct treatment for uncontrolled HIV-associated sensory neuropathy.
3.1.2 Central and Peripheral Neuropathic Pain
Three studies examined the effect of marijuana on chronic neuropathic pain.
Wilsey et al. (2008) examined chronic neuropathic pain from multiple causes in the study entitled, "A Randomized, Placebo-Controlled, Crossover Trial of Cannabis Cigarettes in Neuropathic Pain". The subjects were 32 patients with a variety of neuropathic pain conditions, including 22 with complex regional pain syndrome, 6 with spinal cord injury, 4 with multiple sclerosis, 3 with diabetic neuropathy, 2 with ilioinguinal neuralgia, and 1with lumbosacral plexopathy. All subjects reported a pain intensity of at least 30 on a 0-100 VAS and were allowed to continue taking their regular medications during the study period, which included opioids, antidepressants, anticonvulsants, and NSAIDs. All subjects were required to have experience with marijuana but could not use any cannabinoids for 30 days before study sessions.
The study consisted of three test sessions with an interval of 3-21 days between sessions. Treatment conditions were high-strength marijuana (7% delta-9-THC), low-strength marijuana (3.5% delta-9-THC), and placebo cigarettes, administered through a standardized cued-puff procedure: (1) "light the cigarette" (30 seconds), (2) "get ready" (5 seconds), (3) "inhale" (5 seconds), (4) "hold smoke in lungs" (10 seconds), (5) "exhale," and (6) wait before repeating the puff cycle (40 seconds). Participants took 2 puffs after baseline measurements, 3 puffs an hour later, and 4 puffs an hour after that, for a cumulative dose of 9 puffs per test session.
Hourly assessment periods were scheduled before and after each set of puffs and for 2 additional hours during the recovery period. Plasma cannabinoids were measured at baseline, 5 minutes after the first puff and again at 3 hours after the last puff cycle.
The primary outcome measure was spontaneous pain relief, as measured by a 0-100 point VAS for current pain. Pain unpleasantness was measured on a 0-100 point VAS, and degree of pain relief was measured on a 7-point Patient Global Impression of Change (PGIC) scale. Secondary measures included the Neuropathic Pain Scale (NPS), a 0-100 point VAS for allodynia, and changes in thermal pain threshold. Subjective measures were also evaluated with unipolar 0-100 point VAS for any drug effect, good drug effect, bad drug effect, high, drunk, impaired, stoned, like the drug effect, sedated, confused, nauseated, desire more of the drug, anxious, down, hungry, and bipolar 0-100 point VAS for sad/happy, anxious/relaxed, jittery/calm, bad/good, paranoid/self-assured, fearful/unafraid. Neurocognitive assessments measured attention and concentration, learning and memory, and fine motor speed.
Marijuana produced a reduction in pain compared to placebo, as measured by the pain VAS, the PGIC and on pain descriptors in the NPS, including sharp (P < .001), burning (P < .001), aching (P < .001), sensitive (P = .03), superficial (P < .01) and deep pain (P < .001). Notably, there were no additional benefits from the 7% THC strength of marijuana compared to the 3.5% THC strength, seemingly because of cumulative drug effects over time. There were no changes in allodynia or thermal pain responsivity following administration of either dose of marijuana.
Marijuana at both strengths produced increases on measures of any drug effect, good drug effect, high, stoned, impairment, sedation, confusion, and hunger. The 7% THC marijuana increased anxiety scores and bad drug effect (later in session) compared to placebo. Neither strength of marijuana affected the measures of mood. On neurocognitive measures, both the 3.5% THC and 7% THC marijuana produced impairment in learning and memory, while only the 7% THC marijuana impaired attention and psychomotor speed, compared to placebo. There were no adverse cardiovascular side effects and no subjects dropped out because of an adverse event related to marijuana.
The authors conclude that marijuana may be effective at ameliorating neuropathic pain at doses that induce mild cognitive effects, but that smoking is not an optimum route of administration. The limitations of this study include: inclusion of subjects with many forms of neuropathic pain and maintenance of subjects on other analgesic medication while being tested with marijuana. These limitations make it difficult to conclude that marijuana has analgesic properties on its own and that the actual AEs experienced during the study in response to marijuana are tolerable. The authors compared pain score results by the type of pain condition, with no significant differences found; however, the sample size of this study was small thus a type II error may have been present. Thus, it Start Printed Page 53719 is difficult to determine if any particular subset of neuropathic pain conditions would benefit specifically from marijuana administration. However, the study produced positive results suggesting that marijuana should be studied further as an adjunct treatment for uncontrolled neuropathic pain.
The second study, conducted by Ware et al. (2010) in Canada is entitled, "Smoked cannabis for chronic neuropathic pain: a randomized controlled trial". The subjects were 21 adult patients with neuropathic pain caused by trauma or surgery compounded with allodynia or hyperalgesia, and a pain intensity score greater than 4 on a 10 point VAS. All subjects maintained their current analgesic medication and they were allowed to use acetaminophen for breakthrough pain. Eighteen subjects had previous experience with marijuana but none of them had used marijuana within a year before the study.
The study design used a four-period crossover design, testing marijuana (2.5%, 6.0% and 9.4% THC) and placebo marijuana. The 2.5% and 6.0% doses of marijuana were included to increase successful blinding. Each period was 14 days in duration, beginning with 5 days on the study drug followed by a 9-day washout period. Doses were delivered as 25 mg of marijuana that was smoked in a single inhalation using a titanium pipe. The first dose of each period was self-administered using a standardized puff procedure: (1) Inhale for 5 seconds, (2) hold the smoke in their lungs for 10 seconds, and (3) exhale. Subsequent doses were self-administered in the same manner for a total of three times daily at home on an outpatient basis for the first five days of each period.
The primary measure was an 11-point pain intensity scale, averaged over the 5 day treatment period, which was administered once daily for present, worst, least and average pain intensity during the previous 24 hours. Secondary measures included an acute pain 0-100 point VAS, pain quality assessed with the McGill Pain Questionnaire, sleep assessed with the Leeds Sleep Evaluation Questionnaire, mood assessed with the POMS, quality of life assessed using the EQ-5D health outcome instrument. Subjective measures included 0-100 point VAS scales for high, relaxed, stressed and happy.
Over the first three hours after smoking marijuana, ratings of pain, high, relaxation, stress, happiness and heart rate were recorded. During the five days of each study period, participants were contacted daily to administer questionnaires on pain intensity, sleep, medication and AEs. Subjects returned on the fifth day to complete questionnaires on pain quality, mood, quality of life and assessments of potency. At the end of the study, participants completed final adverse event reports and potency assessments.
The average daily pain intensity was significantly lower on 9.4% THC marijuana (5.4) than on placebo marijuana (6.1) (p = 0.023). The 9.4% THC strength also produced more drowsiness, better sleep, with less anxiety and depression, compared to placebo (all p < 0.05). However, there were no significant differences on POMS scores or on VAS scores for high, happy, relaxed or stressed between THC doses.
The most frequent drug-related adverse events reported in the group receiving 9.4% THC marijuana were headache, dry eyes, burning sensation, dizziness, numbness and cough. Reports of high and euphoria occurred on only three occasions, once in each dose of THC. There were no significant changes in vital signs, heart-rate variability, or renal function. One subject withdrew from the study due to increased pain during administration of 6% THC marijuana.
The authors conclude that smoked marijuana reduces neuropathic pain, improves mood and aids in sleep, but that smoking marijuana is not a preferable route of administration. The limitations of this study include: The lack of information on timing of assessments during the outpatient portion of the study and maintenance of subjects on other analgesic medication while being tested with marijuana. These limitations make it difficult to conclude that marijuana has analgesic properties on its own and that the actual AEs experienced during the study in response to marijuana are tolerable. However, the study produced positive results suggesting that marijuana should be studied further as an adjunct treatment for uncontrolled neuropathic pain.
Wilsey et al. (2013) conducted the most recent study entitled, "Low-Dose Vaporized Cannabis Significantly Improves Neuropathic Pain". This study is the only one in this review that utilized vaporization as a method of marijuana administration. The subjects were 36 patients with a neuropathic pain disorder (CRPS, thalamic pain, spinal cord injury, peripheral neuropathy, radiculopathy, or nerve injury) who were maintained on their current medications (opioids, anticonvulsants, antidepressants, and NSAIDs). Although subjects were required to have a history of marijuana use, they refrained from use of cannabinoids for 30 days before study sessions.
Subjects participated in three sessions in which they received 1.29% or 3.53% THC marijuana or placebo marijuana. The marijuana was vaporized using the Volcano vaporizer and a standardized cued-puff procedure: (1) "hold the vaporizer bag with one hand and put the vaporizer mouthpiece in their mouth" (30 seconds), (2) "get ready" (5 seconds), (3) "inhale" (5 seconds), (4) "hold vapor in lungs" (10 seconds), (5) "exhale and wait" before repeating puff cycle (40 seconds). Subjects inhaled 4 puffs at 60 minutes. At 180 minutes, the vaporizer was refilled with marijuana vapor and subjects were allowed to inhale 4 to 8 puffs using the cued procedure. Thus, cumulative dosing allowed for a range of 8 to12 puffs in total for each session, depending on the subjects desired response and tolerance. The washout time between each session ranged from 3-14 days.
The primary outcome variable was spontaneous pain relief, as assessed using a 0-100 point VAS for current pain. Secondary measures included the Patient Global Impression of Change (PGIC), the Neuropathic Pain Scale (NPS), a 0-100 point VAS for allodynia. Acute pain threshold was measured with a thermal pain model. Subjective measures included 0-100 point unipolar VAS for any drug effect, good drug effect, bad drug effect, high, drunk, impaired, stoned, drug liking, sedated, confused, nauseated, desire more drug, anxious, down and hungry. Bipolar 0-100 point VAS included sad/happy, anxious/relaxed, jittery/calm, bad/good, paranoid/self-assured, and fearful/unafraid. Neurocognitive assessments assessed attention and concentration, learning and memory, and fine motor speed.
A 30% reduction in pain was achieved in 61% of subjects who received the 3.53% THC marijuana, in 57% of subjects who received the 1.29% THC marijuana and in 26% of subjects who received the placebo marijuana (p = 0.002 for placebo vs. 3.53% THC, p = 0.007 for placebo vs 1.29% THC; p > 0.05 1.29% THC vs. 3.53% THC). Both strengths of marijuana significantly decreased pain intensity, unpleasantness, sharpness, and deepness on the NPS, as well as pain ratings on the PGIC, compared to placebo. These effects on pain were maximal with cumulative dosing over the course of the study session, with maximal effects at 180 minutes. There were no effects of marijuana compared to placebo on measures of allodynia or Start Printed Page 53720 thermal pain. Subjects correctly identified the study treatment 63% of the time for placebo, 61% of the time for 1.29% THC, and 89% of the time for 3.53% THC.
On subjective measures, marijuana produced dose-dependent increases compared to placebo on ratings for: any drug effect, good drug effect, drug liking, high, stoned, sedated, confused, and hungry. Both strengths of marijuana produced similar increases in drunk or impaired compared to placebo. In contrast, desire for drug was rated as higher for the 1.29% THC marijuana compared to the 3.53% THC marijuana. There were no changes compared to placebo for bad effect, nauseous, anxiety, feeling down or any of the bipolar mood assessments. There was dose-dependent impairment on learning and memory from marijuana compared to placebo, but similar effects between the two strengths of marijuana on attention.
The authors conclude that vaporization of relatively low doses of marijuana can produce improvements in analgesia in neuropathic pain patients, especially when patients are allowed to titrate their exposure. However, this individualization of doses may account for the general lack of difference between the two strengths of marijuana. No data were presented regarding the total amount of THC consumed by each subject, so it is difficult to determine a proper dose-response evaluation. Additional limitations of this study are the inclusion of subjects with many forms of neuropathic pain and maintenance of subjects on other analgesic medication while being tested with marijuana. These limitations make it difficult to conclude that marijuana has analgesic properties on its own. It is also difficult to determine if any particular subset of neuropathic pain conditions would benefit specifically from marijuana administration. However, the study produced positive results suggesting that marijuana should be studied further as an adjunct treatment for uncontrolled neuropathic pain.
3.2 Appetite Stimulation in HIV
Two randomized, double-blind, placebo-controlled Phase 2 studies examined the effects of smoked marijuana on appetite in HIV-positive subjects (Haney et al., 2005; Haney et al., 2007). Table 2 of the Appendix summarizes both studies.
The first study, conducted by Haney et al. (2005) is entitled, "Dronabinol and marijuana in HIV+ marijuana smokers: acute effects on caloric intake and mood". The subjects were 30 HIV-positive patients who were maintained on two antiretroviral medications and either had clinically significant decreases in lean muscle mass[34] (low-BIA group, n = 15) or normal lean muscle mass (normal-BIA group, n = 15). All subjects had a history of smoking marijuana at least twice weekly for 4 weeks prior to entry into the study. On average, individuals had smoked 3 marijuana cigarettes per day, 5-6 times per week for 10-12 years.
Subjects participated in 8 sessions that tested the acute effects of 0, 10, 20, and 30 mg dronabinol oral capsules and marijuana cigarettes with 0%, 1.8%, 2.8%, and 3.9% THC concentration by weight, using a double-dummy design (with only one active drug per session). The doses of dronabinol are higher than those doses typically prescribed for appetite stimulation in order to help preserve the blinding. There was a one-day washout period between test sessions.
Marijuana was administered using a standardized cued procedure: (1) "light the cigarette" (30 seconds), (2) "prepare" (5 seconds), (3) "inhale" (5 seconds), (4) "hold smoke in lungs" (10 seconds), and (5) "exhale." Each subject smoked three puffs in this manner, with a 40-second interval between each puff.
Caloric intake was used as a surrogate measure for weight gain. Subjects received a box containing a variety of food and beverage items and were told to record consumption of these items following that day's administration of the test drug. Subjective measures included 0-100 point VAS for feel drug effect, good effect, bad effect, take drug again, drug liking, hungry, full, nauseated, thirsty, desire to eat. Neurocognitive measures and vital signs were monitored.
The low BIA group consumed significantly more calories in the 1.8% and 3.9% THC marijuana conditions (p < 0.01) and the 10, 20, and 30 mg dronabinol conditions (p < 0.01) compared with the placebo condition. In contrast, in the normal BIA group, neither marijuana nor dronabinol significantly affected caloric intake. This lack of effect may be accountable, however, by the fact that this group consumed approximately 200 calories more than the low BIA group under baseline conditions.
Ratings of high and good drug effect were increased by all drug treatments in both the low-BIA and normal-BIA groups, except in response to the 10 mg dose of dronabinol. The 3.9% THC marijuana increased ratings of good drug effect, drug liking and desire to smoke again compared with placebo. Ratings of sedation were increased in both groups by 10 and 30 mg dronabinol, and in the normal BIA group by the 2.8% THC marijuana. Ratings of stimulation were increased in the normal BIA group by 2.8% and 3.9% THC marijuana and by 20 mg dronabinol. Increases in ratings of forgetfulness, withdrawn, dreaming, clumsy, heavy limbs, heart pounding, jittery, and decreases in ratings of energetic, social, and talkative were reported in the normal BIA group with 30 mg dronabinol. There were no significant changes in vital signs or performance on neurocognitive measures in response to marijuana. Notably, the time course of subjective effects peaked quickly and declined thereafter for smoked marijuana, while oral dronabinol responses took longer to peak and persisted longer. Additionally, marijuana but not dronabinol produced dry mouth and thirst.
In general, AEs reported in this study were low in both drug conditions for both subject groups. In the low BIA group, nausea was reported by one subject in both the 10 and 20 mg dronabinol conditions, while an uncomfortable level of intoxication was produced by the 30 mg dose in two subjects. There were no AEs reported in this group following marijuana at any dose. In the normal BIA group, the 30 mg dose of dronabinol produced an uncomfortable level of intoxication in three subjects and headache in one subject, while the 3.9% marijuana produced diarrhea in one subject.
The authors conclude that smoked marijuana can acutely increase caloric intake in low BIA subjects without significant cognitive impairment. However, it is possible that the low degree of cognitive impairment reported in this study may reflect the development of tolerance to cannabinoids in this patient population, since all individuals had current histories of chronic marijuana use. Additional limitations in this study include not utilizing actual weight gain as a primary measure. However, the study produced positive results suggesting that marijuana should be studied further as a treatment for appetite stimulation in HIV patients.
A second study conducted by Haney et al. (2007) is entitled, "Dronabinol and marijuana in HIV-positive marijuana smokers: Caloric intake, mood, and sleep". The design of this study was nearly identical to the one conducted by this laboratory in 2005 (see above), but Start Printed Page 53721 there was no stratification of subjects by BIA. The subjects were 10 HIV-positive patients who were maintained on two antiretroviral medications and had a history of smoking marijuana at least twice weekly for 4 weeks prior to entry into the study. On average, individuals had smoked 3 marijuana cigarettes per day, 5 times per week for 19 years.
Subjects participated in 8 sessions that tested the acute effects of 0, 5 and 10 mg dronabinol oral capsules and marijuana cigarettes with 0, 2.0% and 3.9% THC concentration by weight, using a double-dummy design (with 4 sessions involving only one active drug and 4 interspersed placebo sessions). Both drug and placebo sessions lasted for 4 days each, with active drug administration occurring 4 times per day (every 4 hours). Testing occurred in two 16-day inpatient stays. In the intervening outpatient period, subjects were allowed to smoke marijuana prior to re-entry to the study unit for the second inpatient stay.
Marijuana was administered using a standardized cued procedure: (1) "light the cigarette" (30 seconds), (2) "prepare" (5 seconds), (3) "inhale" (5 seconds), (4) "hold smoke in lungs" (10 seconds), and (5) "exhale." Each subject smoked three puffs in this manner, with a 40-second interval between each puff.
Caloric intake was used as a surrogate measure for weight gain, but subjects were also weighed throughout the study (a measure which was not collected in the 2005 study by this group). Subjects received a box containing a variety of food and beverage items and were told to record consumption of these items following that day's administration of the test drug. Subjective measures included 0-100 point VAS for drug effect, good effect, bad effect, take drug again, drug liking, hungry, full, nauseated, thirsty, desire to eat. Neurocognitive measures and vital signs were monitored. Sleep was assessed using both the Nightcap sleep monitoring system and selected VAS measures related to sleep.
Both 5 and 10 mg dronabinol (p < 0.008) and 2.0% and 3.9% THC marijuana (p < 0.01) dose-dependently increased caloric intake compared with placebo. This increase was generally accomplished through increases in incidents of eating, rather than an increase in the calories consumed in each incident. Subjects also gained similar amounts of weight after the highest dose of each cannabinoid treatment: 1.2 kg (2.6 lbs) after 4 days of 10 mg dronabinol, and 1.1 kg (2.4 lbs) after 4 days of 3.9% THC marijuana. The 3.9% THC marijuana dose also increased the desire to eat and ratings of hunger.
Ratings of good drug effect, high, drug liking, and desire to smoke again were significantly increased by 10 mg dronabinol and 2.0% and 3.9% THC marijuana doses compared to placebo. Both marijuana doses increased ratings of stimulated, friendly, and self-confident. The 10 mg dose of dronabinol increased ratings of concentration impairment, and the 2.0% THC marijuana dose increased ratings of anxious. Dry mouth was induced by 10 mg dronabinol (10 mg) and 2.0% THC marijuana. There were no changes in neurocognitive performance or objective sleep measures from administration of either cannabinoid. However, 3.9% THC marijuana increased subjective ratings of sleep.
The authors conclude that both dronabinol and smoked marijuana increase caloric intake and produce weight gain in HIV-positive patients. However, it is possible that the low degree of cognitive impairment reported in this study may reflect the development of tolerance to cannabinoids in this subject population, since all individuals had current histories of chronic marijuana use. This study produced positive results suggesting that marijuana should be studied further as a treatment for appetite stimulation in HIV patients.
3.3 Spasticity in Multiple Sclerosis
Only one randomized, double-blind, placebo-controlled Phase 2 study examined the effects of smoked marijuana on spasticity in MS.
This study was conducted by Corey-Bloom et al. (2012) and is entitled, "Smoked cannabis for spasticity in multiple sclerosis: A randomized, placebo-controlled trial". The subjects were 30 patients with MS-associated spasticity and had moderate increase in tone (score ≥ 3 points on the modified Ashworth scale). Participants were allowed to continue other MS medications, with the exception of benzodiazepines. Eighty percent of subjects had a history of marijuana use and 33% had used marijuana within the previous year.
Subjects participated in two 3-day test sessions, with an 11 day washout period. During each test session they smoked a 4.0% THC marijuana cigarette once per day or a placebo cigarette once per day. Smoking occurred through a standardized cued-puff procedure: (1) Inhalation for 5 seconds, (2) breath-hold and exhalation for 10 seconds, (3) pause between puffs for 45 seconds. Subjects completed an average of four puffs per cigarette.
The primary outcome measure was change in spasticity on the modified Ashworth scale. Additionally, subjects were assessed using a VAS for pain, a timed walk, and cognitive tests (Paced Auditory Serial Addition Test) and AEs.
Treatment with 4.0% THC marijuana reduced subject scores on the modified Ashworth scale by an average of 2.74 points more than placebo (p < 0.0001) and reduced VAS pain scores compared to placebo (p = 0.008). Scores on the cognitive measure decreased by 8.7 points more than placebo (p = 0.003). However, marijuana did not affect scores for the timed walk compared to placebo. Marijuana increased rating of feeling high compared to placebo.
7 subjects did not complete the study due to adverse events (two subjects felt uncomfortably "high", two had dizziness and one had fatigue). Of those 7 subjects who withdrew, 5 had little or no previous experience with marijuana. When the data were re-analyzed to include these drop-out subjects, with the presumption they did not have a positive response to treatment, the effect of marijuana was still significant on spasticity.
The authors conclude that smoked marijuana had usefulness in reducing pain and spasticity associated with MS. It is concerning that marijuana-naïve subjects dropped out of the study because they were unable to tolerate the psychiatric AEs induced by marijuana. The authors suggest that future studies should examine whether different doses can result in similar beneficial effects with less cognitive impact. However, the current study produced positive results suggesting that marijuana should be studied further as an adjunct treatment for spasticity in MS patients.
3.4 Asthma
Tashkin et al. (1974) examined bronchodilation in 10 subjects with bronchial asthma in the study entitled, "Acute Effects of Smoked Marijuana and Oral Δ9-Tetrahydrocannabinol on Specific Airway Conductance in Asthmatic Subjects". The study was a double-blind, placebo-controlled, crossover design. All subjects were clinically stable at the time of the study; four subjects were symptom free, and six subjects had chronic symptoms of mild to moderate severity. Subjects were tested with 0.25ml of isoproterenol HCl prior to the study to ensure they responded to bronchodilator medications. Subjects were not allowed to take bronchodilator medication within 8 hours prior to the study. Previous experience with marijuana was not required for participation in the study, but 7 of the 10 subjects reported Start Printed Page 53722 previous use of marijuana at a rate of less than 1 marijuana cigarette per month. No subjects reported marijuana use within 7 days of the study.
The study consisted of four test sessions with an interval of at least 48 hours between sessions. On two test sessions subjects smoked 7 mg/kg of body weight of either marijuana, with 2% THC concentration by weight, or placebo marijuana. During the other two test sessions, subjects ingested capsules with either 15mg of synthetic THC or placebo. Marijuana was administered using a uniform smoking technique: subjects inhaled deeply for 2-4 seconds, held smoke in lungs for 15 seconds, and resumed normal breathing for approximately 5 seconds. The author did not provide a description of the number of puffs taken at any smoking session. The authors state that the smoking procedure was repeated until the cigarette was consumed, which took approximately 10 minutes.
The outcome measure used was specific airway conductance (SGaw), as calculated using measurements of thoracic gas volume (TGV) and airway resistance (Raw) using a variable-pressure body plethysmograph. Additionally, an assessment of degree of intoxication was administered only to those subjects reporting previous marijuana use. This assessment consisted of subjects rating "how `high' they felt" on a scale of 0-7, 7 representing "the `highest' they had ever felt after smoking marijuana".
Marijuana produced a significant increase of 33-48% in average SGaw compared to both baseline and placebo (P < 0.05). This significant increase in SGaw lasted for at least 2 hours after administration. The average TGV significantly decreased by 4-13% compared to baseline and placebo (P < 0.05). The author stated that all subjects reported feelings of intoxication after marijuana administration.
The authors conclude that marijuana produced bronchodilation in clinically stable asthmatic subjects with minimal to moderate bronchospasms. Study limitations include: inclusion of subjects with varying severity of asthmatic symptoms, use of SGaw to measure lung responses to marijuana administration, and administration of smoke to asthmatic subjects. Smoke delivers a number of harmful substances and is not an optimal delivery symptom, especially for asthmatic patients. FEV1 via spirometry is the gold standard to assess changes in lung function, pre and post asthma treatment, by pharmacotherapy. SGaw has been shown to be a valid tool in bronchoconstriction lung assessment; however, since the FEV1 method was not utilized, it is unclear whether these results would correlate if the FEV1 method had been employed.
3.5 Glaucoma
Two randomized, double-blind, placebo-controlled Phase 2 clinical studies examined smoked marijuana in glaucoma (Crawford and Merritt, 1979; Merritt et al., 1980). In both studies, intraocular pressure (IOP) was significantly reduced 30 minutes after smoking marijuana. Maximal effects occurred 60-90 minutes after smoking, with IOP returning to baseline within 3-4 hours. These two studies were included in the 1999 IOM report on the medical uses of marijuana. Because our independent analysis of these studies concurred with the conclusions from the 1999 IOM report, these studies will not be discussed in further detail in this review. No recent studies have been conducted examining the effect of inhaled marijuana on IOP in glaucoma patients. This lack of recent studies may be attributed to the conclusions made in the 1999 IOM report that while cannabinoids can reduce intraocular pressure (IOP), the therapeutic effects require high doses that produce short-lasting responses, with a high degree of AEs. This high degree of AEs means that the potential harmful effects of chronic marijuana smoking may outweigh its modest benefits in the treatment of glaucoma.
3.6 Conclusions
Of the eleven randomized, double-blind, placebo-controlled Phase 2 clinical studies that met the criteria for review (see Sections 2.2 and 2.3), ten studies administered marijuana through smoking, while one study utilized marijuana vaporization. In these eleven studies, there were five different therapeutic indications: Five examined chronic neuropathic pain, two examined appetite stimulation in HIV patients, two examined glaucoma, one examined spasticity in MS, and one examined asthma.
There are limited conclusions that can be drawn from the data in these published studies evaluating marijuana for the treatment of different therapeutic indications. The analysis relied on published studies, thus information available about protocols, procedures, and results were limited to documents published and widely available in the public domain. The published studies on medical marijuana are effectively proof-of-concept studies. Proof-of-concept studies provide preliminary evidence on a proposed hypothesis regarding a drug's effect. For drugs under development, the effect often relates to a short-term clinical outcome being investigated. Proof-of-concept studies serve as the link between preclinical studies and dose ranging clinical studies. Therefore, proof-of-concept studies are not sufficient to demonstrate efficacy of a drug because they provide only preliminary information about the effects of a drug. Although these studies do not provide evidence that marijuana is effective in treating a specific, recognized disorder, these studies do support future larger well-controlled studies to assess the safety and efficacy of marijuana for a specific medical indication. Overall, the conclusions below are preliminary, based on very limited evidence.
3.6.1 Conclusions for Chronic Neuropathic Pain
In subjects with chronic neuropathic pain who are refractory to other pain treatments, five proof-of-concept studies produced positive results regarding the use of smoked marijuana for analgesia. However, the subjects in these studies continued to use their current analgesic drug regime, and thus no conclusions can be made regarding the potential efficacy of marijuana for neuropathic pain in patients not taking other analgesic drugs. Subjects also had numerous forms of neuropathic pain, making it difficult to identify whether a specific set of symptoms might be more responsive to the effects of marijuana. It is especially concerning that some marijuana-naïve subjects had intolerable psychiatric responses to marijuana exposure at analgesic doses.
3.6.2 Conclusions for Appetite Stimulation in HIV
In subjects who were HIV-positive, two proof-of-concept studies produced positive results with the use of both dronabinol and smoked marijuana to increase caloric intake and produce weight gain in HIV-positive patients. However, the amount of THC in the marijuana tested in these studies is four times greater than the dose of dronabinol typically tested for appetite stimulation (10 mg vs. 2.5 mg; Haney et al., 2005). Thus, it is possible that the low degree of AEs reported in this study may reflect the development of tolerance to cannabinoids in this patient population, since all individuals had current histories of chronic marijuana use. Thus, individuals with little prior exposure to marijuana may not respond similarly and may not be able to tolerate sufficient marijuana to produce appetite stimulation. Start Printed Page 53723
3.6.3 Conclusions for Spasticity in MS
In subjects with MS, a proof of concept study produced positive results using smoked marijuana as a treatment for pain and symptoms associated with treatment-resistant spasticity. The subjects in this study continued to take their current medication regiment, and thus no conclusions can be made regarding the potential efficacy of marijuana when taken on its own. It is also concerning that marijuana-naïve subjects dropped out of the study because they were unable to tolerate the psychiatric AEs induced by marijuana. The authors suggest that future studies should examine whether different doses can result in similar beneficial effects with less cognitive impact.
3.6.4 Conclusions for Asthma
In subjects with clinically stable asthma, a proof of concept study produced positive results of smoked marijuana producing bronchodilation. However, in this study marijuana was administered at rest and not while experiencing bronchospasms. Additionally, the administration of marijuana through smoking introduces harmful and irritating substances to the subject, which is undesirable especially in asthmatic patients. Thus the results suggest marijuana may have bronchodilator effects, but it may also have undesirable adverse effects in subjects with asthma.
3.6.5 Conclusions for Glaucoma
As noted in Sections 3.5, the two studies that evaluated smoked marijuana for glaucoma were conducted decades ago, and they have been thoroughly evaluated in the 1999 IOM report. The 1999 IOM report concludes that while the studies with marijuana showed positive results for reduction in IOP, the effect is short-lasting, requires a high dose, and is associated with many AEs. Thus, the potential harmful effects may outweigh any modest benefit of marijuana for this condition. We agree with the conclusions drawn in the 1999 IOM report.
3.7 Design Challenges for Future Studies
The positive results reported by the studies discussed in this review support the conduct of more rigorous studies in the future. This section discusses methodological challenges that have occurred in clinical studies with smoked marijuana. These design issues should be addressed when larger-scale clinical studies are conducted to ensure that valid scientific data are generated in studies evaluating marijuana's safety and efficacy for a particular therapeutic use.
3.7.1 Sample Size
The ability for results from a clinical study to be generalized to a broader population is reliant on having a sufficiently large study sample size. However, as noted above, all of the 11 studies reviewed in this document were early Phase 2 proof of concept studies for efficacy and safety. Thus, the sample sizes used in these studies were inherently small, ranging from 10 subjects per treatment group (Tashkin et al., 1974; Haney et al., 2007) to 25 subjects per treatment group (Abrams et al., 2007). These sample sizes are statistically inadequate to support a showing of safety or efficacy. FDA's recommendations about sample sizes for clinical trials can be found in the Guidance for Industry: E9 Statistical Principles for Clinical Trials (1998). [35] For example, "the number of subjects in a clinical trial should always be large enough to provide a reliable answer to the questions addressed. This number is usually determined by the primary objective of the trial. The method by which the sample size is calculated should be given in the protocol, together with the estimates of any quantities used in the calculations (such as variances, mean values, response rates, event rates, difference to be detected)." (pg. 21). Other clinical FDA Guidance for Industry[36] may also contain recommendations regarding the appropriate number of subjects that should be investigated for a specific medical indication.
3.7.2 Marijuana Dose Standardization
Dose standardization is critical for any clinical study in order to ensure that each subject receives a consistent exposure to the test drug. The Guidance for Industry: Botanical Drug Products (2004)[37] provides specific information on the development of botanical drug products. Specifically, this guidance includes information about the need for well-characterized and consistent chemistry for the botanical plant product and for consistent and reliable dosing. Specifically for marijuana studies, dose standardization is important because if marijuana leads to plasma levels of cannabinoids that are significantly different between subjects, this variation may lead to differences in therapeutic responsivity or in the prevalence of psychiatric AEs.
In most marijuana studies discussed in this review, investigators use a standardized cued smoking procedure. In this procedure, a subject is instructed to inhale marijuana smoke for 5 seconds, hold the smoke in the lungs for 10 seconds, exhale and breathe normally for 40 seconds. This process is repeated to obtain the desired dose of the drug. However, this procedure may not lead to equivalent exposure to marijuana and its constituent cannabinoids, based on several factors:
- Intentional or unintentional differences in the depth of inhalation may change the amount of smoke in the subject's lungs.
- Smoking results in loss from side stream smoke, such that the entire dose is not delivered to the subject.
- There may be differences in THC concentration along the length of a marijuana cigarette. According to Tashkin et al. (1991), the area of the cigarette closest to the mouth tends to accumulate a higher concentration of THC, but this section of the cigarette is not smoked during a study.
For example, Wilsey et al. (2008) used this standardized smoking procedure. The reported mean (range) of marijuana cigarettes consumed was 550 mg (200-830mg) for the low strength marijuana (3.5% THC) and 490 mg (270-870mg) for the high strength marijuana (7% THC). This wide range of amounts of marijuana cigarette smoked by the individual subjects, even with standardized smoking procedure and controlled number of puffs, supports the issues with delivering consistent doses with smoke marijuana.
In other marijuana studies that do not use a cued smoking procedure, subjects are simply told to smoke the marijuana cigarette over a specific amount of time (usually 10 minutes) without further instruction (Crawford and Merritt, 1979; Merritt et al., 1980; Ellis et al., 2009). The use of a nonstandardized procedure may lead to non-equivalent exposures to marijuana and its constituent cannabinoids between subjects because of additional factors that are not listed above, such as:
- Differences in absorption and drug response if subjects (especially marijuana-naïve ones) are not instructed to hold marijuana smoke in their lungs for a certain period of time. Start Printed Page 53724
- Prolonged periods between puffs may increase loss to side stream smoke.
- Subjects may attempt to smoke the marijuana cigarette in the way they would smoke a tobacco cigarette, which relies primarily on short, shallow puffs.
In both standardized and non-standardized smoking procedures, subjects may seek to control the dose of THC through self-titration (Crawford and Merritt, 1979; Merritt et al., 1980; Tashkin et al., 1974; Abrams et al., 2007; Ellis et al., 2009). Self-titration involves an individual moderating the amount of marijuana smoke inhaled over time in order to obtain a preferred level of psychoactive or clinical response. The ability of an individual to self-titrate by smoking is one reason given by advocates of "medical marijuana" in support of smoking of marijuana rather than through its ingestion via edibles. However, for research purposes, self-titration interferes with the ability to maintain consistent dosing levels between subjects, and thus, valid comparisons between study groups.
All of these factors can make the exact dose of cannabinoids received by a subject in a marijuana study difficult to determine with accuracy. Testing whether plasma levels of THC or other cannabinoids are similar between subjects following the smoking procedure would establish whether the procedure is producing appropriate results. Additionally, studies could be conducted to determine if vaporization can be used to deliver consistent doses of cannabinoids from marijuana plant material. Specifically, vaporization devices that involve the collection of vapors in an enclosed bag or chamber may help with delivery of consistent doses of marijuana. Thus, more information could be collected on whether vaporization is comparable to or different than smoking in terms of producing similar plasma levels of THC in subjects using identical marijuana plant material.
3.7.3 Acute vs. Chronic Therapeutic Marijuana Use
The studies that were reviewed administered the drug for short durations lasting no longer than 5 days (Abrams et al., 2007; Ellis et al., 2009; Ware et al., 2010). Thus all studies examined the short-term effect of marijuana administration for therapeutic purposes. However, many of the medical conditions that have been studied are persistent or expected to last the rest of a patient's life. Therefore, data on chronic exposure to smoked marijuana in clinical studies is needed. In this way, more information will be available regarding whether tolerance, physical dependence, or specific adverse events develop over the course of time with continuing use of therapeutic marijuana.
3.7.4 Smoking as a Route of Administration
As has been pointed out by the IOM and other groups, smoking is not an optimum route of administration for marijuana-derived therapeutic drug products, primarily because introducing the smoke from a burnt botanical substance into the lungs of individuals with a disease state is not recommended when their bodies may be physically compromised. The 1999 IOM report on medicinal uses of marijuana noted that alternative delivery methods offering the same ability of dose titration as smoking marijuana will be beneficial and may limit some of the possible long-term health consequences of smoking marijuana. The primary alternative to smoked marijuana is vaporization, which can reduce exposure to combusted plant material containing cannabinoids. The only study to use vaporization as the delivery method was Wilsey et al. (2013). The results from Wilsey et al. (2013) showed a similar effect of decreased pain as seen in the other studies using smoking as the delivery method (Ware et al., 2010; Wilsey et al., 2008). This similar effect of decrease pain supports vaporization as a possibly viable route to administer marijuana in research, while potentially limiting the risks associated with smoking.
3.7.5 Difficulty in Blinding of Drug Conditions
An adequate and well-controlled clinical study involves double-blinding, where both the subjects and the investigators are unable to tell the difference between the test treatments (typically consisting of at least a test drug and placebo) when they are administered. All of the studies reviewed in this document administered study treatments under double-blind conditions and thus were considered to have an appropriate study design.
However, even under the most rigorous experimental conditions, blinding can be difficult in studies with smoked marijuana because the rapid onset of psychoactive effects readily distinguishes active from placebo marijuana. The presence of psychoactive effects also occurs with other drugs. However, most other drugs have a similar psychoactive effect with substances with similar mechanisms of actions. These substances can be used as positive controls to help maintain blinding to the active drug being tested. Marijuana on the other hand, has a unique set of psychoactive effects which makes the use of appropriate positive controls difficult (Barrett et al., 1995). However, two studies did use Dronabinol as a positive control drug to help maintain blinding (Haney et al., 2005; Haney et al., 2007).
When blinding is done using only placebo marijuana, the ability to distinguish active from placebo marijuana may lead to expectation bias and an alteration in perceived responsivity to the therapeutic outcome measures. With marijuana-experienced subjects, for example, there may be an early recognition of the more subtle cannabinoid effects that can serve as a harbinger of stronger effects, which is less likely to occur with marijuana-naïve subjects. To reduce this possibility, investigators have tested doses of marijuana other than the one they were interested in experimentally to maintain the blind (Ware et al., 2010).
Blinding can also be compromised by differences in the appearance of marijuana plant material based on THC concentration. Marijuana with higher concentrations of THC tends to be heavier and seemingly darker, with more "tar-like" substance. Subjects who have experience with marijuana have reported being able to identify marijuana from placebo cigarettes by sight alone when the plant material in a cigarette was visible (Tashkin et al., 1974; Ware et al., 2010). Thus, to maintain a double-blind design, many studies obscure the appearance of plant material by closing both ends of the marijuana cigarette and placing it in in an opaque plastic tube.
While none of these methods to secure blinding may be completely effective, it is important to reduce bias as much as possible to produce consistent results between subjects under the same experimental conditions.
3.7.6 Prior Marijuana Experience
Marijuana use histories in test subjects may influence outcomes, related to both therapeutic responsivity and psychiatric AEs. Marijuana-naïve subjects may also experience a marijuana drug product as so aversive that they would not want to use the drug product. Thus, subjects' prior experience with marijuana may affect the conduct and results of studies.
Most of the studies reviewed in this document required that subjects have a history of marijuana use (see tables in Appendix that describe specific requirements for each study). However, in studies published in the scientific literature, the full inclusion criteria with Start Printed Page 53725 regard to specific amount of experience with marijuana may not be provided. For those studies that do provide inclusion criteria, acceptable experience with marijuana can range from once in a lifetime to use multiple times a day.
The varying histories of use might affect everything from scores on adverse event measures, safety measures, or efficacy measures. Additionally, varying amounts of experience can impact cognitive effect measures assessed during acute administration studies. For instance, Schreiner and Dunn (2012) contend cognitive deficits in heavy marijuana users continue for approximately 28 days after cessation of smoking. Studies requiring less than a month of abstinence prior to the study may still see residual effects of heavy use at baseline and after placebo marijuana administration, thus showing no significant effects on cognitive measures. However, these same measurements in occasional or naïve marijuana users may demonstrate a significant effect after acute marijuana administration. Therefore, the amount of experience and the duration of abstinence of marijuana use are important to keep in mind when analyzing results for cognitive and other adverse event measures. Lastly, a study population with previous experience with marijuana may underreport the incidence and severity of adverse events. Because most studies used subjects with prior marijuana experience, we are limited in our ability to generalize the results, especially for safety measures, to marijuana naïve populations.
Five of 11 studies reviewed in this document included both marijuana-naïve and marijuana-experienced subjects (Corey-Bloom et al., 2012; Ellis et al., 2009; Ware et al., 2010; Merritt et al., 1980; Tashkin et al., 1974). Since the number of marijuana-naïve subjects in these studies was low, it was not possible to conduct a separate analysis compared to experienced users. However, systematically evaluating the effect of marijuana experience on study outcomes is important, since many patients who might use a marijuana product for a therapeutic use will be marijuana-naïve.
Research shows that marijuana-experienced subjects have a higher ability to tolerate stronger doses of oral dronabinol than marijuana-naïve subjects (Haney et al., 2005). Possibly, this increased tolerance is also the case when subjects smoke or vaporize marijuana. Thus, studies could be conducted that investigate the role of marijuana experience in determining tolerability of and responses to a variety of THC concentrations in marijuana.
3.7.7 Inclusion and Exclusion Criteria
For safety reasons, all clinical studies have inclusion and exclusion criteria that restrict the participation of individuals with certain medical conditions. For studies that test marijuana, these criteria may be based on risks associated with exposure to smoked material and the effects of THC. Thus, most studies investigating marijuana require that subjects qualify for the study based on restrictive symptom criteria such that individuals do not have other symptoms that may be known to interact poorly with cannabinoids.
Similarly, clinical studies with marijuana typically exclude individuals with cardiac or pulmonary problems, as well as psychiatric disorders. These exclusion criteria are based on the well-known effects of marijuana smoke to produce increases in heart rate and blood pressure, lung irritation, and the exacerbation of psychiatric disturbances in vulnerable individuals. Although these criteria are medically reasonable for research protocols, it is likely that future marijuana products will be used in patients who have cardiac, pulmonary or psychiatric conditions. Thus, individuals with these conditions should be evaluated, whenever possible.
Additionally, all studies reviewed in this document allowed the subjects to continue taking their current regimen of medications. Thus all results evaluated marijuana as an adjunct treatment for each therapeutic indication.
3.7.8 Number of Female Subjects
A common problem in clinical research is the limited number of females who participate in the studies. This problem is present in the 11 studies reviewed in this document, in which one study did not include any female subjects (Ellis et al., 2009), and three studies had a low percentage of female subjects (Abrams et al., 2007; Haney et al., 2005; Haney et al., 2007). However, each of these four studies investigated an HIV-positive patient population, where there may have been a larger male population pool from which to recruit compared to females.
Since there is some evidence that the density of CB1 receptors in the brain may vary between males and females (Crane et al., 2012), there may be differing therapeutic or subjective responsivity to marijuana. Studies using a study population that is equal parts male and female may show whether and how the effects of marijuana differ between male and female subjects.
4. References
1999. Marijuana and Medicine: Assessing the Science Base. Washington, DC: National Academy Press.
Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, Benowitz NL, Bredt BM, Kosel B, Aberg JA, Deeks SG, Mitchell TF, Mulligan K, Bacchetti P, McCune JM, and Schambelan M. 2003. Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized, placebo-controlled clinical trial. Annals of Internal Medicine 139 (4): 258-266.
Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, Kelly ME, Rowbotham MC, and Petersen KL. 2007. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology 68 (7): 515-521.
Appendino G, Chianese G, Taglialatela-Scafati O. 2011. Cannabinoids: occurrence and medicinal chemistry. Curr Med Chem. 18(7):1085-99.
Barrett RL, Wiley JL, Balster RL, and Martin BR. 1995. Pharmacological specificity of Δ9-tetrahydrocannabinol discrimination in rats. Psychopharmacology 118(4): 419-424.
Chait LD, and Pierri J. 1989. Some physical characteristics of NIDA marijuana cigarettes. Addictive Behaviors 14 (1): 61-67.
Chang AE, Shiling DJ, Stillman RC, Godlberg NH, Seipp CA, Barofsky I, Simon RM, and Rosenberg SA. 1979. Delta-9-tetrahydrocannabinol as an antiemetic in cancer patients receiving high-dose methotrexate. Annals of Internal Medicine 91: 819-824.
Corey-Bloom J, Wolfson T, Gamst A, Jin S, Marcotte TD, Bentley H, and Gouaux B. 2012. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. Canadian Medical Association Journal 184 (10): 1143-1150.
Crane NA, Schuster RM, Fusar-Poli P, and Gonzalez R. 2012. Effects of Cannabis on Neurocognitive Functioning: Recent Advances, Neurodevelopmental Influences, and Sex Differences. Neuropsychology Review.
Crawford WJ, and Merritt JC. 1979. Effects of tetrahydrocannabinol on arterial and intraocular hypertension. International Journal of Clinical Pharmacology and biopharmacy 17 (5): 191-196.
Ellis RJ, Toperoff W, Vaida F, Van Den Brande G, Gonzales J, Gouaux B, Bentley H, and Atkinson JH. 2009. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 34 (3): 672-680.
Flom MC, Adams AJ, and Jones RT. 1975. Marijuana smoking and reduced pressure in human eyes: drug action or epiphenomenon? Investigative Opthalmology 14(1): 52-55.
Foltin RW, Brady JV, and Fischamn MW. 1986. Behavioral analysis of marijuana effects on food intake in humans. Pharmacology Biochemistry and Behavior 25: 577-582. Start Printed Page 53726
Foltin RW, Fischman MW, and Byrne MF. 1988. Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory. Appetite 11: 1-14.
Greenberg HS, Werness SA, Pugh JE, Andrus RO, Anderson DJ, and Domino EF. 1994. Short-term effects of smoking marijuana on balance in patients with multiple sclerosis and normal volunteers. Clinical Pharmacology and Therapeutics 55 (3): 324-328.
Greenwald MK and Stitzer ML. 2000. Antinociceptive, subjective, and behavioral effects of smoked marijuana in humans. Drug and Alcohol Dependence 59: 261-275.
Haney M, Gunderson EW, Rabkin J, Hart CL, Vosburg SK, Comer SD, and Foltin RW. 2007. Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood, and sleep. Journal of Acquired Immune Deficiency Syndromes (1999) 45 (5): 545-554.
Haney M, Rabkin J, Gunderson E, and Foltin RW. 2005. Dronabinol and marijuana in HIV(+) marijuana smokers: acute effects on caloric intake and mood. Psychopharmacology 181 (1): 170-178.
Hill SY, Schwin R, Goodwin DW, and Powell BJ. 1974. Marihuana and pain. Journal of Pharmacology and Experimental Therapeutics 188(2): 415-418.
Jampel H. 2010. American glaucoma society position statement: marijuana and the treatment of glaucoma. Journal of Glaucoma 19 (2): 75-76.
Merritt JC, Crawford WJ, Alexander PC, Anduze AL, and Gelbart SS. 1980. Effect of marihuana on intraocular and blood pressure in glaucoma. Ophthalmology 87 (3): 222-228.
Milstein SL, MacCannell KL, Karr GW, and Clark S. 1974. Marijuana produced changes in cutaneous sensitivity and affect: users and non-users. Pharmacology Biochemistry and Behavior 2:367-374.
Milstein SL, MacCannell K, Karr G, and Clark S. 1975. Marijuana-produced changes in pain tolerance: Experiences and non-experienced subjects. Int. Pharmacopsychiat 10: 177-182.
Naftali T, Schleider LB, Dotan I, Lansky EP, Benjaminov FS, and Konikoff FM. 2013. Cannabis induces a clinical response in patients with Crohn's disease: A prospective placebo-controlled study. Clinical Gastroenterology and Hepatology 11: 1276-1280.
Russo E, Mathre ML, Byrne A, Velin R, Bach PJ, Sanchez-Ramos J, and Kirlin KA. 2002. Chronic Cannabis Use in the Compassionate Investigational New Drug Program: An Examination of Benefits and Adverse Effects of Legal Clinical Cannabis. Journal of Cannabis Therapeutics 2 (1): 3-57.
Soderpalm AHV, Schuster A, and de Wit H. 2001. Antiemetic efficacy of smoked marijuana subjective and behavioral effects on nausea induced by syrup of ipecac. Pharmacology Biochemistry and Behavior 69: 343-350.
Tashkin DP, Gliederer F, Rose J, Chang P, Hui KK, Yu JL, and Wu TC. 1991. Tar, CO and delta 9THC delivery from the 1st and 2nd halves of a marijuana cigarette. Pharmacology Biochemistry and Behavior 40 (3): 657-661.
Tashkin DP, Shapiro BJ, Lee YE, Harper CE. 1975. Effects of smoked marijuana in experimentally induced asthma. American Review of Respiratory Disease 112: 377-386.
Wallace M, Schulteis G, Atkinson JH, Wolfson T, Lazzaretto D, Bentley H, Gouaux B, and Abramson I. 2007. Dose-dependent effects of smoked cannabis on capsaicin-induced pain and hyperalgesia in healthy volunteers. Anesthesiology 107 (5): 785-796.
Ware MA, Wang T, Shapiro S, Robinson A, Ducruet T, Huynh T, Gamsa A, Bennett GJ, and Collet JP. 2010. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. Canadian Medical Association Journal 182 (14): E694-E701.
Wilsey B, Marcotte T, Tsodikov A, Millman J, Bentley H, Gouaux B, and Fishman S. 2008. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J. Pain 9 (6): 506-521.
Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, and Donaghe H. 2013. Low-dose vaporized cannabis significantly improves neuropathic pain. J. Pain 14(2):136-48.
Start Printed Page 53727

Start Printed Page 53728

Start Printed Page 53729
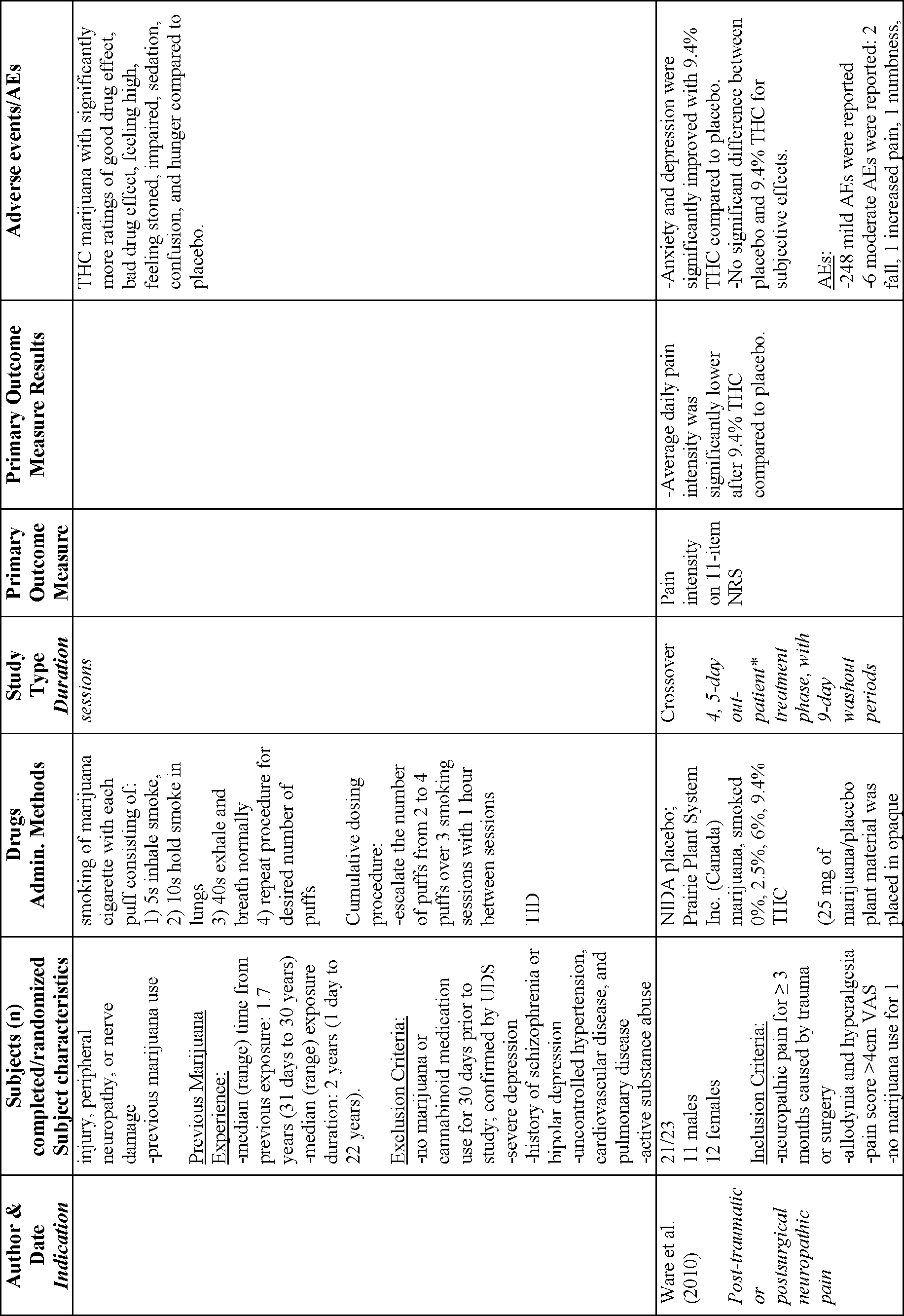
Start Printed Page 53730

Start Printed Page 53731

Start Printed Page 53732

Start Printed Page 53733

Start Printed Page 53734

Start Printed Page 53735

Start Printed Page 53736

Start Printed Page 53737

Start Printed Page 53738

Start Printed Page 53739
U.S. Department of Justice—Drug Enforcement Administration
Schedule of Controlled Substances: Maintaining Marijuana in Schedule I of the Controlled Substances Act
Background, Data, and Analysis: Eight Factors Determinative of Control and Findings Pursuant to 21 U.S.C. 812(b)
Prepared by: Office of Diversion Control, Drug and Chemical Evaluation Section, Washington, DC 20537
July 2016
Background
On November 30, 2011, Governors Lincoln D. Chafee of Rhode Island and Christine O. Gregoire of Washington submitted a petition to the Drug Enforcement Administration (DEA) to initiate proceedings for a repeal of the rules or regulations that place marijuana[38] in schedule I of the Controlled Substances Act (CSA). The petition requests that marijuana[39] and "related items" be rescheduled in schedule II of the CSA. The petitioners claim that:
1. Cannabis has accepted medical use in the United States;
2. Cannabis is safe for use under medical supervision;
3. Cannabis for medical purposes has a relatively low potential for abuse, especially in comparison with other schedule II drugs.
The DEA accepted this petition for filing on January 30, 2012.
The Attorney General may by rule transfer a drug or other substance between schedules of the CSA if she finds that such drug or other substance has a potential for abuse, and makes the findings prescribed by 21 U.S.C. 812(b) for the schedule in which such drug is to be placed. 21 U.S.C. 811(a)(1). The Attorney General has delegated this responsibility to the Acting Administrator of the DEA. 28 CFR 0.100(b).
In accordance with 21 U.S.C. 811(b), after gathering the necessary data, the DEA submitted the petition and necessary data to the Department of Health and Human Services (HHS) on June 11, 2013, and requested that HHS provide a scientific and medical evaluation and scheduling recommendation for marijuana. In documents dated June 3 and June 25, 2015, the acting Assistant Secretary for Health of the HHS[40] recommended to the DEA that marijuana continue to be controlled in Schedule I of the CSA, and provided to the DEA its scientific and medical evaluation titled "Basis for the Recommendation for Maintaining Marijuana in Schedule I of the Controlled Substances Act." The HHS's recommendations are binding on the DEA as to scientific and medical matters. 21 U.S.C. 811(b).
Before initiating proceedings to reschedule a substance, the CSA requires the Acting Administrator to determine whether the HHS scheduling recommendation, scientific and medical evaluation, and "all other relevant data" constitute substantial evidence that the drug should be rescheduled as proposed. 21 U.S.C. 811(b). The Acting Administrator must determine whether there is substantial evidence to conclude that the drug meets the criteria for placement in another schedule based on the criteria set forth in 21 U.S.C. 812(b). The CSA requires that both the DEA and the HHS consider the eight factors specified by Congress in 21 U.S.C. 811(c). This document lays out those considerations and is organized according to the eight factors. As DEA sets forth in detail below, the evidence shows:
1. Actual or relative potential for abuse. Marijuana has a high potential for abuse. Preclinical and clinical data show that it has reinforcing effects characteristic of drugs of abuse. National databases on actual abuse show marijuana is the most widely abused drug, including significant numbers of substance abuse treatment admissions. Data on marijuana seizures show widespread availability and trafficking.
2. Scientific evidence of its pharmacological effect. The scientific understanding of marijuana, cannabinoid receptors, and the endocannabinoid system continues to be studied and elucidated. Marijuana produces various pharmacological effects, including subjective (e.g., euphoria, dizziness, disinhibition), cardiovascular, acute and chronic respiratory, immune system, and prenatal exposure effects, as well as behavioral and cognitive impairment.
3. Current scientific knowledge. There is no currently accepted medical use for marijuana in the United States. Marijuana sources are derived from numerous cultivated strains and may have different levels of Δ9-THC and other cannabinoids. Under the five-element test for currently accepted medical use discussed in more detail below and upheld by the Court of Appeals for the District of Columbia in Alliance for Cannabis Therapeutics v. DEA, 15 F.3d 1131, 1135 (D.C. Cir. 1994) (hereinafter "ACT"), there is no complete scientific analysis of marijuana's chemical components; there are not adequate safety studies; there are not adequate and well-controlled efficacy studies; there is not a consensus of medical opinion concerning medical applications of marijuana; and the scientific evidence regarding marijuana's safety and efficacy is not widely available. To date, scientific and medical research has not progressed to the point that marijuana has a currently accepted medical use, even under conditions where its use is severely restricted.
4. History and current pattern of abuse. Marijuana continues to be the most widely used illicit drug. In 2014, there were 22.2 million current users. There were also 2.6 million new users, most of whom were less than 18 years of age. During the same period, marijuana was the most frequently identified drug exhibit in federal, state, and local forensic laboratories.
5. Scope, duration, and significance of abuse. Abuse of marijuana is widespread and significant. In 2014, for example, an estimated 6.5 million people aged 12 or older used marijuana on a daily or almost daily basis over a 12-month period. In addition, a significant proportion of all admissions for substance abuse treatment are for marijuana/hashish as their primary drug of abuse. In 2013, 16.8% of all such admissions—281,991 over the course of the year—were for primary marijuana/hashish abuse.
6. Risk, if any, to public health. Together with the health risks outlined in terms of pharmacological effects above, public health risks from acute use of marijuana include impaired psychomotor performance, impaired driving, and impaired performance on tests of learning and associative Start Printed Page 53740 processes. Chronic use of marijuana poses a number of other risks to the public health including physical as well as psychological dependence.
7. Psychic or physiological dependence liability. Long-term, heavy use of marijuana can lead to physical dependence and withdrawal following discontinuation, as well as psychic or psychological dependence. In addition, a significant proportion of all admissions for treatment for substance abuse are for primary marijuana abuse; in 2013, 16.8% of all admissions were for primary marijuana/hashish abuse, representing 281,991 individuals.
8. Immediate precursor. Marijuana is not an immediate precursor of any controlled substance.
As specified in 21 U.S.C. 812(b)(1), in order for a substance to be placed in schedule I, the Acting Administrator must find that:
A. The drug or other substance has a high potential for abuse.
B. The drug or other substance has no currently accepted medical use in treatment in the United States.
C. There is a lack of accepted safety for use of the drug or other substance under medical supervision.
To be classified in another schedule under the CSA (e.g., II, III, IV, or V), a substance must have a "currently accepted medical use in treatment in the United States." 21 U.S.C. 812(b)(2)-(5). A substance also may be placed in schedule II if it is found to have "a currently accepted medical use with severe restrictions." 21 U.S.C. 812(b)(2). If a controlled substance has no such currently accepted medical use, it must be placed in schedule I. See Notice of Denial of Petition, 66 FR 20038 (Apr. 18, 2001) ("Congress established only one schedule—schedule I—for drugs of abuse with `no currently accepted medical use in treatment in the United States' and `lack of accepted safety for use . . . under medical supervision.' ").
A drug that is the subject of an approved new drug application (NDA) or abbreviated new drug application (ANDA) under Federal Food, Drug, and Cosmetic Act (21 U.S.C. 355), is considered to have a currently accepted medical use in treatment in the United States for purposes of the CSA. The HHS stated in its review, however, that FDA has not approved any NDA for marijuana for any indication.
In the absence of NDA or ANDA approval, DEA has established a five-element test for determining whether the drug has a currently accepted medical use in treatment in the United States. Under this test, a drug will be considered to have a currently accepted medical use only if the following five elements are satisfied:
1. The drug's chemistry is known and reproducible;
2. There are adequate safety studies;
3. There are adequate and well-controlled studies proving efficacy;
4. The drug is accepted by qualified experts; and
5. The scientific evidence is widely available.
(57 FR 10499, 10506 (March 26, 1992)). See also ACT, 15 F.3d at 1135.
As discussed in Factor 3, below, HHS concluded, and DEA agrees, that the scientific evidence is insufficient to demonstrate that marijuana has a currently accepted medical use under the five-element test. The evidence was insufficient in this regard also when the DEA considered petitions to reschedule marijuana in 1992 (57 FR 10499),[41] in 2001 (66 FR 20038), and in 2011 (76 FR 40552).[42] Little has changed since 2011 with respect to the lack of clinical evidence necessary to establish that marijuana has a currently accepted medical use. No studies have scientifically assessed the efficacy and full safety profile of marijuana for any specific medical condition.
The limited existing clinical evidence is not adequate to warrant rescheduling of marijuana under the CSA. To the contrary, the data in this scheduling review document show that marijuana continues to meet the criteria for schedule I control under the CSA for the following reasons:
1. Marijuana has a high potential for abuse.
2. Marijuana has no currently accepted medical use in treatment in the United States.
3. Marijuana lacks accepted safety for use under medical supervision.
Factor 1: The Drug's Actual or Relative Potential for Abuse
Marijuana is the most commonly abused illegal drug in the United States. It is also the most commonly used illicit drug by high school students in the United States. Further, marijuana is the most frequently identified drug by state, local and federal forensic laboratories. Marijuana's main psychoactive ingredient, Δ[9] -tetrahydrocannabinol (Δ[9] -THC),[43] is an effective reinforcer in laboratory animals, including primates and rodents. These animal studies both predict and support the observations that marijuana produces reinforcing effects in humans. Such reinforcing effects can account for the repeated abuse of marijuana.
A. Indicators of Abuse Potential
The HHS has concluded in its document, "Basis for the Recommendation for Maintaining Marijuana in Schedule I of the Controlled Substances Act," that marijuana has a high potential for abuse. The finding of "abuse potential" is critical for control under the Controlled Substances Act (CSA). Although the term is not defined in the CSA, guidance in determining abuse potential is provided in the legislative history of the Act (Comprehensive Drug Abuse Prevention and Control Act of 1970, H.R. Rep. No. 91-1444, 91st Cong., Sess. 2 (1970), reprinted in 1970 U.S.C.C.A.N. 4566, 4603). Accordingly, the following items are indicators that a drug or other substance has potential for abuse:
- There is evidence that individuals are taking the drug or drugs containing such a substance in amounts sufficient to create a hazard to their health or to the safety of other individuals or of the community; or
- There is significant diversion of the drug or drugs containing such a substance from legitimate drug channels; or
- Individuals are taking the drug or drugs containing such a substance on their own initiative rather than on the basis of medical advice from a practitioner licensed by law to administer such drugs in the course of his professional practice; or
- The drug or drugs containing such a substance are new drugs so related in their action to a drug or drugs already listed as having a potential for abuse to make it likely that the drug will have the same potentiality for abuse as such drugs, thus making it reasonable to assume that there may be significant diversions from legitimate channels, significant use contrary to or without medical advice, or that it has a substantial capability of creating hazards to the health of the user or to the safety of the community.
Of course, evidence of actual abuse of a substance is indicative that a drug has a potential for abuse.
In its recommendation, the HHS analyzed and evaluated data on marijuana as applied to each of the above four criteria. The analysis presented in the recommendation (HHS, 2015) is discussed below:
1. There is evidence that individuals are taking the drug or drugs containing such a substance in amounts sufficient to create a hazard to their health or to Start Printed Page 53741 the safety of other individuals or of the community.
The HHS stated that some individuals are taking marijuana in amounts sufficient to create a hazard to their health and to the safety of other individuals and the community. Data from national databases on actual abuse of marijuana support the idea that a large number of individuals use marijuana. In its recommendation (HHS, 2015), the HHS presented data from the National Survey on Drug and Health (NSDUH) of the Substance Abuse and Mental Health Services Administration (SAMHSA) and the Monitoring the Future (MTF) survey of the National Institute on Drug Abuse (NIDA), and the DEA has since updated this information. The most recent data from SAMHSA's NSDUH in 2014 reported that marijuana was the most used illicit drug. Among Americans aged 12 years and older, an estimated 22.2 million Americans used marijuana within the past month according to the 2014 NSDUH. In 2004, an estimated 14.6 million individuals reported using marijuana within the month prior to the study. The estimated rates in 2014 thus reflect an increase of approximately 7.6 million individuals over a 10-year period. According to the 2013 NSDUH report, an estimated 19.8 million individuals reported using marijuana. Thus, over a period of one year (2013 NSDUH-2014 NSDUH), there was an estimated increase of 2.4 million individuals in the United States using marijuana.
The results from the 2015 Monitoring the Future survey of 8th, 10th, and 12th grade students indicate that marijuana was the most widely used illicit drug in these age groups. Current monthly use was 6.5% of 8th graders, 14.8% of 10th graders, and 21.3% of 12th graders. The Treatment Episode Data Set (TEDS) in 2013 reported that marijuana abuse was the primary factor in 16.8 percent of non-private substance-abuse treatment facility admissions. In 2011, SAMHSA's Drug Abuse Warning Network (DAWN) reported that marijuana was mentioned in 36.4% (455,668 out of approximately 1.25 million) of illicit drug-related Emergency Department (ED) visits.
Data on the extent and scope of marijuana abuse are presented under Factors 4 and 5 of this analysis. Discussion of the health effects of marijuana is presented under Factor 2, and the assessment of risk to the public health posed by acute and chronic marijuana abuse is presented under Factor 6 of this analysis.
2. There is significant diversion of the drug or drugs containing such a substance from legitimate drug channels.
In accordance with the CSA, the only lawful source of marijuana in the United States is that produced and distributed for research purposes under the oversight of NIDA and in conformity with United States obligations under the Single Convention on Narcotic Drugs.[44] The HHS stated that there is a lack of significant diversion from legitimate drug sources, but that this is likely due to high availability of marijuana from illicit sources. Marijuana is not an FDA-approved drug product. Neither a New Drug Application (NDA) nor a Biologics License Application (BLA) has been approved for marketing in the United States. However, the marijuana used for nonclinical and clinical research represents a very small amount of the total amount of marijuana available in the United States and therefore information about marijuana diversion from legitimate sources is limited or not available.
The DEA notes that the magnitude of the demand for illicit marijuana is evidenced by information from a number of databases presented under Factor 4. Briefly, marijuana is the most commonly used illegal drug in the United States. It is also the most commonly used illicit drug by American high schoolers. Marijuana is the most frequently identified drug in state, local, and federal forensic laboratories, with increasing amounts of both domestically grown and of illicitly smuggled marijuana.
Given that marijuana has long been the most widely trafficked and abused controlled substance in the United States, and that all aspects of such illicit activity are entirely outside of the closed system of distribution mandated by the CSA, it may well be the case that there is little thought given to diverting marijuana from the small supplies produced for legitimate research purposes. Thus, the lack of data indicating diversion of marijuana from legitimate channels to the illicit market is not indicative of a lack of potential for abuse of the drug.
3. Individuals are taking the drug or drugs containing such a substance on their own initiative rather than on the basis of medical advice from a practitioner licensed by law to administer such drugs in the course of his professional practice.
The HHS stated that the FDA has not evaluated or approved an NDA or BLA for marijuana for any therapeutic indication. Consistent with federal law, therefore, an individual legitimately can take marijuana based on medical advice from a practitioner only by participating in research that is being conducted under an Investigational New Drug (IND) application. The HHS noted that there are several states as well as the District of Columbia which have passed laws allowing for individuals to use marijuana for purported "medical" use under certain circumstances, but data are not available yet to determine the number of individuals using marijuana under these state laws. Nonetheless, according to 2014 NSDUH data, 22.2 million American adults currently use marijuana (SAMHSA, 2015a). Based on the large number of individuals who use marijuana and the lack of an FDA-approved drug product, the HHS concluded that the majority of individuals using marijuana do so on their own initiative rather than by following medical advice from a licensed practitioner.
4. The drug or drugs containing such a substance are new drugs so related in their action to a drug or drugs already listed as having a potential for abuse to make it likely that the drug will have the same potentiality for abuse as such drugs, thus making it reasonable to assume that there may be significant diversions from legitimate channels, significant use contrary to or without medical advice, or that it has a substantial capability of creating hazards to the health of the user or to the safety of the community.
Marijuana and its primary psychoactive ingredient, Δ9-THC, are controlled substances in schedule I under the CSA.
The HHS stated that one approved, marketed drug product contains synthetic Δ9-THC, also known as dronabinol, and another approved, marketed drug product contains a cannabinoid-like synthetic compound that is structurally related to Δ9-THC, the main active component in marijuana. Both products are controlled under the CSA.
Marinol is a schedule III drug product containing synthetic Δ9-THC (dronabinol) formulated in sesame oil in soft gelatin capsules. Marinol was approved by the FDA in 1985 for the treatment of nausea and vomiting associated with cancer chemotherapy in patients who did not respond to conventional anti-emetic treatments. In 1992, FDA approved Marinol for the treatment of anorexia associated with weight loss in patients with acquired immunodeficiency syndrome (AIDS). Marinol was originally placed into schedule II and later rescheduled to schedule III under the CSA due to the Start Printed Page 53742 low reports of abuse relative to marijuana.
Cesamet is a drug product containing the schedule II substance nabilone, a synthetic substance structurally related to Δ9-THC. Cesamet was approved for marketing by the FDA in 1985 for the treatment of nausea and vomiting associated with cancer chemotherapy. All other naturally occurring cannabinoids in marijuana and their synthetic equivalents with similar chemical structure and pharmacological activity are already included as schedule I drugs under the CSA.
B. Abuse Liability Studies
In addition to the indicators suggested by the CSA's legislative history, data as to preclinical and clinical abuse liability studies, as well as actual abuse, including clandestine manufacture, trafficking, and diversion from legitimate sources, are considered in this factor.
Abuse liability evaluations are obtained from studies in the scientific and medical literature. There are many preclinical measures of a drug's effects that when taken together provide an accurate prediction of the human abuse liability. Clinical studies of the subjective and reinforcing effects in humans and epidemiological studies provide quantitative data on abuse liability in humans and some indication of actual abuse trends. Both preclinical and clinical studies have clearly demonstrated that marijuana and Δ9-THC possess the attributes associated with drugs of abuse: They function as a positive reinforcer to maintain drug-seeking behavior, they function as a discriminative stimulus, and they have dependence potential.
Preclinical and most clinical abuse liability studies have been conducted with the psychoactive constituents of marijuana, primarily Δ9-THC and its metabolite, 11-hydroxy-Δ9-THC. Δ9-THC's subjective effects are considered to be the basis for marijuana's abuse liability. The following studies provide a summary of that data.
1. Preclinical Studies
Δ9-THC, the primary psychoactive component in marijuana, is an effective reinforcer in laboratory animals, including primates and rodents, as these animals will self-administer Δ9-THC. These animal studies both predict and support the observations that Δ9-THC, whether smoked as marijuana or administered by other routes, produces reinforcing effects in humans. Such reinforcing effects can account for the repeated abuse of marijuana.
a. Drug Discrimination Studies
The drug discrimination paradigm is used as an animal model of human subjective effects (Solinas et al., 2006) and is a method where animals are able to indicate whether a test drug is able to produce physical or psychological changes similar to a known drug of abuse. Animals are trained to press one bar (in an operant chamber) when they receive a known drug of abuse and another bar when they receive a placebo. When a trained animal receives a test drug, if the drug is similar to the known drug of abuse, it will press the bar associated with the drug.
Discriminative stimulus effects of Δ9-THC have specificity for the pharmacological effects of cannabinoids found in marijuana (Balster and Prescott, 1992; Browne and Weissman, 1981; Wiley et al., 1993; Wiley et al., 1995). As mentioned by the HHS, the discriminative stimulus effects of cannabinoids appear to be unique because abused drugs of other classes including stimulants, hallucinogens, opioids, benzodiazepines, barbiturates, NMDA antagonists, and antipsychotics do not fully substitute for Δ9-THC.
Laboratory animals including monkeys (McMahon et al., 2009), mice (McMahon et al., 2008), and rats (Gold et al., 1992) are able to discriminate cannabinoids from other drugs and placebo. The major active metabolite of Δ9-THC, 11-hydroxy-Δ9-THC, generalizes to Δ9-THC (Browne and Weissman, 1981). In addition, according to the HHS, twenty-two other cannabinoids found in marijuana also substitute for Δ9-THC. At least one cannabinoid, CBD, does not substitute for Δ9-THC in rats (Vann et al., 2008).
b. Self-Administration Studies
Animal self-administration behavior associated with a drug is a commonly used method for evaluating if the drug produces rewarding effects and for predicting abuse potential (Balster, 1991; Balster and Bigelow, 2003). Drugs that are self-administered by animals are likely to produce rewarding effects in humans. As mentioned in the HHS review document, earlier attempts to demonstrate self-administration of Δ9-THC were unsuccessful and confounded by diet restrictions, animal restraint, and known analgesic activity of Δ9-THC at testing doses (Tanda and Goldberg, 2003; Justinova et al., 2003). Self-administration of Δ9-THC was first demonstrated by Tanda et al. (2000). Tanda et al. (2000) showed that squirrel monkeys that were initially trained to self-administer cocaine (30 µg/kg, i.v.) self-administered 2 µg/kg Δ9-THC (i.v.) and at a rate of 30 injections per one hour session. Tanda et al. (2000) used a lower dose of Δ9-THC that was rapidly delivered (0.2 ml injection over 200 ms) than in previous self-administration studies such that analgesic activity of Δ9-THC was not a confounding factor. The authors also stated that the doses were comparable to those doses used by humans who smoke marijuana. A CB1 receptor antagonist (SR141716) blocked this rewarding effect of THC.
Justinova et al. (2003) were able to demonstrate self-administration of Δ9-THC in drug-naïve squirrel monkeys (no previous exposure to other drugs). The authors tested the monkeys with several doses of Δ9-THC (1, 2, 4, 8, and 16 µg/kg, i.v.) and found that the maximal rates of self-administration were observed with the 4 µg/kg/infusion. Subsequently, Braida et al. (2004) reported that rats will self-administer Δ9-THC when delivered intracerebroventricularly (i.c.v.), but only at the lowest doses tested (0.01-0.02 µg/infusion, i.c.v.).
Self-administration behavior with Δ9-THC was found to be antagonized in rats and squirrel monkeys by rimonabant (SR141716A, CB1 antagonist) and the opioid antagonists (naloxone and naltrexone) (Tanda et al., 2000; Braida et al., 2004; Justinova et al., 2004).
c. Conditioned Place Preference Studies
Conditioned place preference (CPP) is a behavioral assay where animals are given the opportunity to spend time in two distinct environments: one where they previously received a drug and one where they received a placebo. If the drug is reinforcing, animals in a drug-free state will choose to spend more time in the environment paired with the drug when both environments are presented simultaneously.
CPP has been demonstrated with Δ9-THC in rats but only at low doses (0.075-1.0 mg/kg, i.p.; Braida et al., 2004). Rimonabant (0.25-1.0 mg/kg, i.p.) and naloxone (0.5-2.0 mg/kg, i.p.) antagonized Δ9-THC-mediated CPP (Braida et al., 2004). However, in another study with rats, rimonabant was demonstrated to induce CPP at doses ranging from 0.25-3.0 mg/kg (Cheer et al., 2000). Mice without µ-opioid receptors did not exhibit CPP to Δ9-THC (paired with 1 mg/kg Δ9-THC, i.p.) (Ghozland et al., 2002).
2. Clinical Studies
In its scientific review (HHS, 2015), the HHS provided a list of common subjective psychoactive responses to cannabinoids based on information from several references (Adams and Martin, 1996; Gonzalez, 2007; Hollister, 1986; Start Printed Page 53743 Hollister, 1988; Institute of Medicine, 1982). Furthermore, Maldonado (2002) characterized these subjective responses as pleasurable to most humans and are generally associated with drug-seeking and/or drug-taking. Later studies (Scherrer et al., 2009; Zeiger et al., 2010) reported that high levels of positive psychoactive effects correlate with increased marijuana use, abuse, and dependence. The list of the common subjective psychoactive effects provided by the HHS (HHS, 2015) is presented below:
(1) Disinhibition, relaxation, increased sociability, and talkativeness.
(2) Increased merriment and appetite, and even exhilaration at high doses.
(3) Enhanced sensory perception, which can generate an increased appreciation of music, art, and touch.
(4) Heightened imagination, which can lead to a subjective sense of increased creativity.
(5) Initial dizziness, nausea, tachycardia, facial flushing, dry mouth, and tremor.
(6) Disorganized thinking, inability to converse logically, time distortions, and short-term memory impairment.
(7) Ataxia and impaired judgment, which can impede driving ability or lead to an increase in risk-taking behavior.
(8) Illusions, delusions, and hallucinations that intensify with higher doses.
(9) Emotional lability, incongruity of affect, dysphoria, agitation, paranoia, confusion, drowsiness, and panic attacks, which are more common in inexperienced or high-dosed users.
The HHS mentioned that marijuana users prefer higher concentrations of the principal psychoactive component (Δ9-THC) over lower concentrations. In a clinical study with marijuana users (n = 12, usage ranged from once a month to 4 times a week), subjects were given a choice of 1.95% Δ9-THC marijuana or 0.63% Δ9-THC marijuana after sampling both marijuana cigarettes in two choice sessions. The marijuana cigarette with high THC was chosen in 21 out of 24 choice sessions or 87.5% of the time (Chait and Burke, 1994). Furthermore, in a double-blind study, frequent marijuana users (n = 11, usage at least 2 times per month with at least 100 occasions) when given a low-dose of oral Δ9-THC (7.5 mg) were able to distinguish the psychoactive effects better than occasional users (n = 10, no use within the past 4 years with 10 or fewer lifetime uses) and also experienced fewer sedative effects (Kirk and de Wit, 1999).
Marijuana has also been recognized by scientific experts to have withdrawal symptoms (negative reinforcement) following moderate and heavy use. As discussed further in Factor 7, the DEA notes that the American Psychiatric Association's (APA) Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) included a list of withdrawal symptoms following marijuana [cannabis] use (DSM-5, 2013).
C. Actual Abuse of Marijuana—National Databases Related to Marijuana Abuse and Trafficking
Marijuana continues to be the most widely used illicit drug. Evidence of actual abuse can be defined by episodes/mentions in databases indicative of abuse/dependence. The HHS provided in its recommendation (HHS, 2015) information relevant to actual abuse of marijuana including data results from the National Survey on Drug Use and Health (NSDUH), a Monitoring the Future (MTF) survey, the Drug Abuse Warning Network (DAWN), and the Treatment Episode Data Set (TEDS). These data sources provide quantitative information on many factors related to abuse of a particular substance, including incidence and patterns of use, and profile of the abuser of specific substances. The DEA is providing updated information from these databases in this discussion. The DEA also includes data on trafficking and illicit availability of marijuana from DEA databases including the National Forensic Laboratory Information System (NFLIS) and the National Seizure System (NSS), formerly the Federal-wide Drug Seizure System (FDSS), as well as other sources of data specific to marijuana, including the Potency Monitoring Project and the Domestic Cannabis Eradication and Suppression Program (DCE/SP).
1. National Survey on Drug Use and Health (NSDUH)
The National Survey on Drug Use and Health (NSDUH) is conducted annually by the Department of Health and Human Service's Substance Abuse and Mental Health Services Administration (SAMHSA). SAMHSA is the primary source of estimates of the prevalence and incidence of pharmaceutical drugs, illicit drugs, alcohol, and tobacco use in the United States. The survey is based on a nationally representative sample of the civilian, non-institutionalized population 12 years of age and older. The survey excludes homeless people who do not use shelters, active military personnel, and residents of institutional group quarters such as jails and hospitals.
According to the 2014 NSDUH report, marijuana was the most commonly used and abused illicit drug. That data showed that there were 22.2 million people who were past month users (8.4%) among those aged 12 and older in the United States. (Note: NSDUH figures on marijuana use include hashish use; the relative proportion of hashish use to marijuana use is very low.) Marijuana had the highest rate of past-year dependence or abuse in 2014. The NSDUH report estimates that 3.0 million people aged 12 or older used an illicit drug for the first time in 2014; a majority (70.3%) of these past year initiates reported that their first drug used was marijuana. Among those who began using illicit drugs in the past year, 65.6%, 70.3%, and 67.6% reported marijuana as the first illicit drug initiated in 2012, 2013, and 2014 respectively. In 2014, the average age of marijuana initiates among 12- to 49-year-olds was 18.5 years. These usage rates and demographics are relevant in light of the risks presented.
Marijuana had the highest rate of past year dependence or abuse of any illicit drug in 2014. The 2014 NSDUH report stated that 4.2 million persons were classified with substance dependence or abuse of marijuana in the past year (representing 1.6% of the total population aged 12 or older, and 59.0% of those classified with illicit drug dependence or abuse) based on criteria specified in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV).
Among past year marijuana users age 12 or older, 18.5% used marijuana on 300 or more days within the previous 12 months in 2014. This translates into 6.5 million people using marijuana on a daily or almost daily basis over a 12-month period, significantly more than the estimated 5.7 million daily or almost daily users in just the year before. Among past month marijuana users, 41.6% (9.2 million) used the drug on 20 or more days in the past month, a significant increase from the 8.1 million who used marijuana 20 days or more in 2013.
2. Monitoring the Future (MTF)
Monitoring the Future (MTF) is an ongoing study which is funded under a series of investigator-initiated competing research grants from the National Institute on Drug Abuse (NIDA). MTF tracks drug use trends among American adolescents in the 8th, 10th, and 12th grades. According to its 2015 survey results, marijuana was the most commonly used illicit drug, as was the case in previous years. Approximately 6.5% of 8th graders, Start Printed Page 53744 14.8% of 10th graders, and 21.3% of 12th graders surveyed in 2015 reported marijuana use during the past month prior to the survey. A number of high school students in 2015 also reported daily use in the past month, including 1.1%, 3.0%, and 6.0% of 8th, 10th, and 12th graders, respectively.
3. Drug Abuse Warning Network (DAWN), Emergency Department (ED) Visits
The Drug Abuse Warning Network (DAWN) is a public health surveillance system that monitors drug-related hospital emergency department (ED) visits to track the impact of drug use, misuse, and abuse in the United States. For the purposes of DAWN, the term "drug abuse" applies if the following conditions are met: (1) The case involved at least one of the following: use of an illegal drug, use of a legal drug contrary to directions, or inhalation of a non-pharmaceutical substance; and (2) the substance was used for one of the following reasons: Because of drug dependence, to commit suicide (or attempt to commit suicide), for recreational purposes, or to achieve other psychic effects. Importantly, many factors can influence the estimates of ED visits, including trends in overall use of a substance as well as trends in the reasons for ED usage. For instance, some drug users may visit EDs for life-threatening issues while others may visit to seek care for detoxification because they needed certification before entering treatment. Additionally, DAWN data do not distinguish the drug responsible for the ED visit from other drugs that may have been used concomitantly. As stated in a DAWN report, "Since marijuana/hashish is frequently present in combination with other drugs, the reason for the ED visit may be more relevant to the other drug(s) involved in the episode."
In 2011, marijuana was involved in 455,668 ED visits out of 2,462,948 total ED visits involving all abuse or misuse in the United States and out of 1.25 million visits involving abuse or misuse of illicit drugs (excluding alcohol-related visits), as estimated by DAWN. This is lower than the number of ED visits involving cocaine (505,224) and higher than the number of ED visits involving heroin (258,482) and stimulants (e.g., amphetamine, methamphetamine) (159,840). Visits involving the other major illicit drugs, such as MDMA, GHB, LSD and other hallucinogens, PCP, and inhalants, were much less frequent, comparatively.
In young patients, marijuana is the illicit drug most frequently involved in ED visits, according to DAWN estimates, with 240.2 marijuana-related ED visits per 100,000 population ages 12 to 17, 443.8 per 100,000 population ages 18 to 20, and 446.9 per 100,000 population ages 21 to 24.
4. Treatment Episode Data Set (TEDS) System
The Treatment Episode Data Set (TEDS) system is part of the SAMHSA Drug and Alcohol Services Information System and is a national census of annual admissions to state licensed or certified, or administratively tracked, substance abuse treatment facilities. The TEDS system contains information on patient demographics and substance abuse problems of admissions to treatment for abuse of alcohol and/or drugs in facilities that report to state administrative data systems. For this database, the primary substance of abuse is defined as the main substance of abuse reported at the time of admission. TEDS also allows for the recording of two other substances of abuse (secondary and tertiary).
In 2011, the TEDS system included 1,928,792 admissions to substance abuse treatment; in 2012 there were 1,801,385 admissions; and in 2013 there were 1,683,451 admissions. Marijuana/hashish was the primary substance of abuse for 18.3% (352,397) of admissions in 2011; 17.5% (315,200) in 2012; and 16.8% (281,991) in 2013. Of the 281,991 admissions for marijuana/hashish treatment in 2013, 24.3% used marijuana/hashish daily. Among those treated for marijuana/hashish as the primary substance in 2013, 27.4% were ages 12 to 17 years and 29.7% were ages 18 to 24 years. Those admitted for marijuana/hashish were mostly male (72.6%) and non-Hispanic (82.2%). Non-hispanic whites (43.2%) represented the largest ethnic group of marijuana admissions.
5. Forensic Laboratory Data
Data on marijuana seizures from federal, state, and local forensic laboratories have indicated that there is significant trafficking of marijuana. The National Forensic Laboratory System (NFLIS) is a program sponsored by the Drug Enforcement Administration's Office of Diversion Control. NFLIS systematically collects drug identification results and associated information from drug exhibits encountered by law enforcement and analyzed in federal, state, and local forensic laboratories. NFLIS is a comprehensive information system that includes data from 278 individual forensic laboratories that report more than 91% of the drug caseload in the U.S. NFLIS captures data for all drugs and chemicals identified and reported by forensic laboratories. More than 1,700 unique substances are represented in the NFLIS database.
Data from NFLIS showed that marijuana was the most frequently identified drug in federal, state, and local laboratories from January 2004 through December 2014. Marijuana accounted for between 29.47% and 34.84% of all drug exhibits analyzed annually during that time frame (Table 1).
Start Printed Page 53745

Since 2004, the total number of reports of marijuana and the amount of marijuana encountered federally has remained high (see data from Federal-wide Drug Seizure System and Domestic Cannabis Eradication and Suppression Program below).
6. Federal-Wide Drug Seizure System
The Federal-wide Drug Seizure System (FDSS) contains information about drug seizures made within the jurisdiction of the United States by the Drug Enforcement Administration, the Federal Bureau of Investigation, United States Customs and Border Protection, and United States Immigration and Customs Enforcement. It also records maritime seizures made by the United States Coast Guard. Drug seizures made by other Federal agencies are included in the FDSS database when drug evidence custody is transferred to one of the agencies identified above. FDSS is now incorporated into the National Seizure System (NSS), which is a repository for information on clandestine laboratory and contraband (chemicals and precursors, currency, drugs, equipment and weapons). FDSS reports total federal drug seizures [in kilograms (kg)] of substances such as cocaine, heroin, MDMA, methamphetamine, and cannabis (marijuana and hashish). The yearly volume of cannabis seized (Table 2), consistently exceeding a thousand metric tons per year, shows that cannabis is very widely trafficked in the United States.

7. Potency Monitoring Project
The University of Mississippi's Potency Monitoring Project (PMP), through a contract with the National Institute on Drug Abuse (NIDA), analyzes and compiles data on the Δ9-THC concentrations of marijuana, hashish and hash oil samples provided by DEA regional laboratories and by state and local police agencies. After 2010, PMP has analyzed only marijuana samples provided by DEA regional laboratories. As indicated in Figure 1, the percentage of Δ9-THC increased from 1995 to 2010 with an average THC content of 3.75% in 1995 and 9.53% in 2010. In examining marijuana samples only provided by DEA laboratories, the average Δ9-THC content was 3.96% in 1995 in comparison to 11.16% in 2015.
Start Printed Page 53746

8. The Domestic Cannabis Eradication and Suppression Program
The Domestic Cannabis Eradication and Suppression Program (DCE/SP) was established in 1979 to reduce the supply of domestically cultivated marijuana in the United States. The program was designed to serve as a partnership between federal, state, and local agencies. Only California and Hawaii were active participants in the program at its inception. However, by 1982 the program had expanded to 25 states and by 1985 all 50 states were participants. Cannabis is cultivated in remote locations and frequently on public lands and illicitly grown in all states. Data provided by the DCE/SP (Table 3) show that in the United States in 2014, there were 3,904,213 plants eradicated in outdoor cannabis cultivation areas compared to 2,597,798 plants in 2000. Significant quantities of marijuana were also eradicated from indoor cultivation operations. There were 396,620 indoor plants eradicated in 2014 compared to 217,105 eradicated in 2000.
Start Printed Page 53747

The recent statistics from these various surveys and databases show that marijuana continues to be the most commonly used illicit drug, with considerable rates of heavy abuse and dependence. They also show that marijuana is the most readily available illicit drug in the United States.
Petitioners' Major Comments in Relation to Factor 1 and the Government's Responses
(1) In Exhibit B, the petitioners compared the effects of marijuana to currently controlled schedule II substances and made repeated claims about the comparative effects.
The HHS noted that comparisons between marijuana and schedule II substances are difficult because of differences in the actions of different pharmacological classes of schedule II drugs in the CSA. The HHS notes that schedule II substances include stimulant-like drugs (e.g., cocaine, amphetamine), opioids (e.g., fentanyl, oxycodone), depressant drugs (e.g., pentobarbital), dissociative anesthetics (e.g., phencyclidine), and naturally occurring plant components (e.g., coca leaves and poppy straw). The mechanism of action of Δ9-THC and marijuana, which act primarily through the cannabinoid receptors (discussed further in Factor 2) are completely different from the above-mentioned classes of schedule II substances. The HHS concludes that the differences in the mechanisms of action in the various classes of schedule II substances make it inappropriate to compare the range of those substances with marijuana.
Furthermore, as noted by the HHS, many substances scheduled under the CSA are evaluated within the context of drug development using data submitted under a New Drug Application (NDA). However, the petitioners have not identified a specific indication for use of marijuana and therefore the HHS notes that an appropriate comparator based on indication cannot be identified.
(2) The petitioners indicated that the actual or relative potential of abuse of marijuana is low. The petitioners state, "Some researchers claim that cannabis is not particularly addictive. Experts assert that cannabis's addictive potential parallels caffeine's." (Exhibit B, page 19, lines 20-21). Furthermore, petitioners stated that, "Cannabis use indicates a lower likelihood of addiction and abuse potential as compared to other substances." (Exhibit B, page 22, lines 12-13).
Under the CSA, for a substance to be placed in schedule II, III, IV, or V, it must have a currently accepted medical use in treatment in the United States.[45] As DEA has previously stated, Congress established only one schedule, schedule I, for drugs of abuse with "no currently accepted medical use in treatment in the United States." 76 FR 40552 (2011). Thus, any attempt to compare the relative abuse potential of schedule I substance to that of a substance in another schedule is inconsequential since a schedule I substance must remain in schedule I until it has been found to have a currently accepted medical use in treatment in the United States.
Moreover, the petitioners failed to review the indicators of abuse potential, as discussed in the legislative history of the CSA. The petitioners did not use data on marijuana usage, diversion, psychoactive properties, and dependence in their evaluation of marijuana abuse potential. The HHS and the DEA discuss those indicators above in this factor. HHS's evaluation of the full range of data led HHS and DEA to conclude that marijuana has a high potential for abuse.
The petitioners, based on their review of a survey by Gore and Earleywine (2007), concluded that marijuana has a low abuse potential. Gore and Earleywine surveyed 746 mental health professionals and asked them to rate the addictiveness (based on a seven-point scale) of several drugs (heroin, nicotine, cocaine/crack, oxycodone, methamphetamine, amphetamine, caffeine, alcohol, and marijuana). The petitioners stated that the health professionals rated marijuana as least addictive of the drugs surveyed. The DEA notes that the survey cited by the petitioners is based on subjective opinions from health professionals.
(3) The petitioners mentioned that many of the cannabinoids in marijuana decrease the psychoactive effects of Δ9-THC, and therefore marijuana lacks sufficient abuse potential for placement into schedule I. Further, the petitioners mentioned on page 4 in Exhibit B (lines 11-15), "While the DEA considers cannabis a schedule I drug, it classifies dronabinol (Marinol) as schedule III. Dronabinol is 100 percent THC and is Start Printed Page 53748 potentially very psychoactive. Natural cannabis typically would be no more than 15 percent THC by weight. Thus it is inconsistent that cannabis, with 15 percent weight THC, remains a [s]chedule I drug, while dronabinol, at 100 percent THC, is schedule III."
The HHS addressed this issue by indicating that the modulating effects of the other cannabinoids in marijuana on Δ9-THC have not been demonstrated in controlled studies. The HHS and the DEA also note that the determination of the abuse potential of a substance considers not only psychoactive effects but also chemistry, pharmacology, pharmacokinetics, usage patterns, and diversion history among other measures.
Marinol (dronabinol in sesame oil) was rescheduled from schedule II to schedule III on July 2, 1999 (64 FR 35928, DEA 1999). In assessing Marinol, HHS compared Marinol to marijuana on several aspects of abuse potential and found that major differences between the two, such as formulation, availability, and usage, contribute to differences in abuse potential. The psychoactive effects from smoking are generally more rapid and intense than those that occur through oral administration (HHS, 2015; Wesson and Washburn, 1990; Hollister and Gillespie, 1973). Therefore, as concluded by both the HHS and the DEA, the delayed onset of action and longer duration of action from an oral dose of Marinol may contribute in limiting the abuse potential of Marinol relative to marijuana, which is most often smoked. The HHS also stated that the extraction and purification of dronabinol from the encapsulated sesame oil mixture of Marinol is highly complex and difficult, and that the presence of sesame oil mixture may preclude the smoking of Marinol-laced cigarettes.
Furthermore, marijuana and Marinol show significant differences in actual abuse and illicit trafficking. There have been no reports of abuse, diversion, or public health risks due to Marinol. In contrast, 22.2 million American adults report currently using marijuana (SAMHSA, 2015a). The DEA database, NFLIS, showed that marijuana was the most frequently identified drug in state and local forensic laboratories from January 2001 to December 2014 and indicates the high availability of marijuana. The differences in composition, actual abuse, and diversion contribute to the differences in scheduling between marijuana and Marinol.
Additionally, the FDA approved a New Drug Application (NDA) for Marinol, indicating a legitimate medical use for Marinol in the United States and allowing for Marinol to be rescheduled into schedule II and subsequently into schedule III of the CSA. The HHS mentioned that marijuana and Marinol differ on a wide variety of factors and these differences are major reasons for differential scheduling of marijuana and Marinol. Marijuana, as discussed more fully in Factors 3 and 6, does not have a currently accepted medical use in the United States, is highly abused, and has a lack of accepted safety.
Factor 2: Scientific Evidence of the Drug Pharmcological Effects, if Known
The HHS stated that there are large amounts of scientific data on the neurochemistry, mechanistic effects, toxicology, and pharmacology of marijuana. A scientific evaluation, as conducted by the HHS and the DEA, of marijuana's neurochemistry, human and animal behavioral pharmacology, central nervous system effects, and other pharmacological effects (e.g., cardiovascular, immunological effects) is presented below.
Neurochemistry
Marijuana contains numerous constituents such as cannabinoids that have a variety of pharmacological actions. The petition defined marijuana as including all cannabis cultivated strains. The HHS stated that different marijuana samples derived from various cultivated strains may differ in their chemical constituents including Δ9-THC and other cannabinoids. Therefore marijuana products from different strains will have different biological and pharmacological effects. The chemical constituents of marijuana are discussed further in Factor 3.
The primary site of action for cannabinoids such as Δ9-THC is at the cannabinoid receptor. Two cannabinoid receptors, CB1 and CB2, have been identified and characterized (Battista et al., 2012; Piomelli, 2005) and are G-protein-coupled receptors. Activation of these inhibitory G-protein-coupled receptors inhibits adenylate cyclase activity, which prevents conversion of ATP to cyclic AMP. Cannabinoid receptor activation also results in inhibition of N- and P/Q-type calcium channels and activates inwardly rectifying potassium channels (Mackie et al., 1995; Twitchell et al., 1997). The HHS mentioned that inhibition of N-type calcium channels decreases neurotransmitter release and this may be the underlying mechanism in the ability of cannabinoids to inhibit acetylcholine, norepinephrine and glutamate from specific areas of the brain. These cellular actions may underlie the antinociceptive and psychoactive effects of cannabinoids. Δ9-THC acts as an agonist at cannabinoid receptors.
CB1 receptors are primarily found in the central nervous system and are located mainly in the basal ganglia, hippocampus and cerebellum of the brain (Howlett et al., 2004). CB1 receptors are also located in peripheral tissues such as the immune system (De Petrocellis and Di Marzo, 2009), but the concentration of CB1 receptors there is considerably lower than in the central nervous system (Herkenham et al., 1990; 1992). CB2 receptors are found primarily in the immune system and predominantly in B lymphocytes and natural killer cells (Bouaboula et al., 1993). CB2 receptors are also found in the central nervous system, primarily in the cerebellum and hippocampus (Gong et al., 2006).
Two endogenous ligands to the cannabinoid receptors, anandamide and arachidonyl glycerol (2-AG), were identified in 1992 (Devane et al., 1992) and 1995 (Mechoulam et al., 1995), respectively. Anandamide is a low-efficacy agonist (Brievogel and Childers, 2000) and 2-AG is a high efficacy agonist (Gonsiorek et al., 2000) to the cannabinoid receptors. These endogenous ligands are present in both the central nervous system and in the periphery (HHS, 2015).
Δ9-THC and cannabidiol (CBD) are two of the major cannabinoids in marijuana. Δ9-THC is the major psychoactive cannabinoid (Wachtel et al., 2002). Δ9-THC has similar affinity for CB1 and CB2 receptors and acts as a weak agonist at CB2 receptors. The HHS indicated that activation of CB1 receptors mediates psychotropic effects of cannabinoids. CBD has low affinity for both CB1 and CB2 receptors. CBD has antagonistic effects at CB1 receptors, and some inverse agonistic properties at CB2 receptors.
Animal Behavioral Effects
Animal abuse potential studies (drug discrimination, self-administration, conditioned place preference) are discussed more fully in Factor 1. Briefly, it was consistently demonstrated that Δ9-THC, the primary psychoactive component in marijuana, and other cannabinoids in marijuana have a distinct drug discriminative profile. In addition, animals self-administer Δ9-THC, and Δ9-THC in low doses produces conditioned place preference. Start Printed Page 53749
Central Nervous System Effects
Psychoactive Effects
The clinical psychoactive effects of marijuana are discussed more fully in Factor 1. Briefly, the psychoactive effects from marijuana use are considered pleasurable and associated with drug-seeking or drug-taking (HHS, 2015; Maldonado, 2002). Further, it was noted by HHS that marijuana users prefer higher concentrations of the principal psychoactive component (Δ9-THC) over lower concentrations (HHS, 2015).
Studies have evaluated psychoactive effects of THC in the presence of high CBD, CBC, or CBN ratios. Even though some studies suggest that CBD may decrease some of Δ9-THC's psychoactive effects, the HHS found that the ratios of CBD to Δ9-THC administered in the studies were not comparable to the amounts found in marijuana used by most people (Dalton et al., 1976; Karniol et al., 1974; Zwardi et al., 1982). In fact, the CBD ratios in these studies are significantly higher than the CBD found in most marijuana currently found on the streets (Mehmedic et al., 2010). HHS indicated that most of the marijuana available on the street has a high THC and low CBD content and therefore any lessening of THC's psychoactive effects by CBD will not occur for most marijuana users (HHS, 2015). Dalton et al. (1976) reported that when volunteers smoked cigarettes with a ratio of 7 CBD to 1 Δ9-THC (0.15 mg/kg CBD and 0.025 mg/kg Δ9-THC), there was a significant decrease in ratings of acute subjective effects and achieving a "high" in comparison to smoking Δ9-THC alone. In oral administration studies, the subjective effects and anxiety produced by combination of CBD and THC in a ratio of at least 1:2 CBD to Δ9-THC (15, 30, 60 mg CBD to 30 mg Δ9-THC; Karniol et al., 1974) or a ratio of 2:1 CBD to Δ9-THC (1 mg/kg CBD to 0.5 mg/kg Δ9-THC; Zuardi et al., 1982) are less than those produced by Δ9-THC administered alone.
In one study (Ilan et al., 2005), the authors calculated the naturally occurring concentrations of CBC and CBD in marijuana cigarettes with either 1.8 or 3.6% Δ9-THC by weight. The authors varied the concentrations of CBC and CBD for each concentration of Δ9-THC in the marijuana cigarettes. Administrations in healthy marijuana users (n=23) consisted of either: (1) Low CBC (0.1% by weight) and low CBD (0.2% by weight); (2) high CBC (0.5% by weight) and low CBD; (3) low CBC and high CBD (1.0% by weight); or (4) high CBC and high CBD and the users were divided into low Δ9-THC (1.8% by weight) and high Δ9-THC (3.6% by weight) groups. Subjective psychoactive effects were significantly greater for all groups in comparison to placebo and there were no significant differences in effects among the treatments (Ilan et al., 2005).
The HHS also referred to a study with Δ9-THC and cannabinol (CBN) (Karniol et al., 1975). In this study, oral administration of either 12.5, 25, or 50 mg CBN combined with 25 mg Δ9-THC (ratio of at least 1:2 CBN to Δ9-THC) significantly increased subjective psychoactive ratings of Δ9-THC compared to Δ9-THC alone (Karniol et al., 1975).
Behavioral Impairment
Several factors may influence marijuana's behavioral effects including the duration (chronic or short term), frequency (daily, weekly, or occasionally), and amount of use (heavy or moderate). Researchers have examined how long behavioral impairments persist following chronic marijuana use. These studies used self-reported histories of exposure duration, frequency, and amount of marijuana use, and administered several performance and cognitive tests at different time points following marijuana abstinence. According to HHS, behavioral impairments may persist for up to 28 days of abstinence in chronic marijuana users.
Psychoactive effects of marijuana can lead to behavioral impairment including cognitive decrements and decreased ability to operate motor vehicles (HHS, 2015). Block et al. (1992) evaluated cognitive measures in 48 healthy male subjects following smoking a marijuana cigarette that contained 2.57% or 19 mg Δ9-THC by weight or placebo. Each subject participated in eight sessions (four sessions with marijuana; four sessions with placebo) and several cognitive and psychomotor tests were administered (e.g. verbal recall, facial recognition, text learning, reaction time). Marijuana significantly impaired performances in most of these cognitive and psychomotor tests (Block et al., 1992).
Ramaekers et al. (2006) reported that in 20 recreational users of marijuana, acute administration of 250 µg/kg and 500 µg/kg Δ9-THC in smoked marijuana resulted in dose-dependent impairments in cognition, motor impulsivity, motor control (tracking impairments), and risk taking. In another study (Kurzthaler et al., 1999), when 290 µg/kg Δ9-THC was administered via a smoked marijuana cigarette in 30 healthy volunteers with no history of substance abuse there were significant impairments of motor speed and accuracy. Furthermore, administration of 3.95% Δ9-THC in a smoked marijuana cigarette increased the latency in a task of simulated braking in a vehicle (Liguori et al., 1998). The HHS noted that the motor impairments reported in these studies (Kurzthaler et al., 1999; Liguori et al., 1998) are critical skills needed for operating a vehicle.
As mentioned in the HHS document, some studies examined the persistence of the behavioral impairments immediately after marijuana administration. Some of marijuana's acute effects may still be present for at least 24 hours after the acute psychoactive effects have subsided. In a brief communication, Heishmann et al. (1990) reported that there were cognitive impairments (digit recall and arithmetic tasks) in two out of three experienced marijuana smokers for 24 hours after smoking marijuana cigarettes containing 2.57% Δ9-THC. However, Fant et al. (1998) evaluated subjective effects and performance measures for up to 25 hours in 10 healthy males after exposure to either 1.8% or 3.6% Δ9-THC in marijuana cigarettes. Peak decrements in subjective and performance measures were noted within 2 hours of marijuana exposure but there were minimal residual alterations in subjective or performance measures at 23-25 hours after exposure.
Persistence of behavioral impairments following repeated and chronic use of marijuana has also been investigated and was reviewed in the HHS document (HHS, 2015). In particular, researchers examined how long behavioral impairments last following chronic marijuana use. In studies examining persistence of effects in chronic and heavy marijuana users, there were significant decrements in cognitive and motor function tasks in all studies of up to 27 days, and in most studies at 28 days (Solowij et al., 2002; Messinis et al., 2006; Lisdahl and Price, 2012; Pope et al., 2002; Bolla et al., 2002; Bolla et al., 2005). In studies that followed heavy marijuana users for longer than 28 days and up to 20 years of marijuana abstinence, cognitive and psychomotor impairments were no longer detected (Fried et al., 2005; Lyons et al., 2004; Tait et al., 2011). For example, Fried et al. (2005) reported that after 3 months of abstinence from marijuana, any deficits in intelligence (IQ), memory, and processing speeds following heavy marijuana use were no longer observed (Fried et al., 2005). In a meta-analysis that examined non-acute and long-lasting effects of marijuana, any deficits in neurocognitive performance that were observed within the first month Start Printed Page 53750 were no longer apparent after approximately one month of abstinence (Schreiner and Dunn, 2012). HHS further notes that in moderate marijuana users deficits in decision-making skills were not observed after 25 days of abstinence and additionally IQ, immediate memory and delayed memory skills were not significantly impacted as observed with heavy and chronic marijuana users (Fried et al., 2005; HHS, 2015).
As mentioned in the HHS document (HHS, 2015), the intensity and persistence of neurological impairment from chronic marijuana use also may be dependent on the age of first use. In two separate smaller scale studies (less than 100 participants per exposure group), Fontes et al. (2011) and Gruber et al. (2012) compared neurological function in early onset (chronic marijuana use prior to age 15 or 16) and late onset (chronic marijuana use after age 15 or 16) heavy marijuana users and found that there were significant deficits in executive neurological function in early onset users which were not observed or were less apparent in late onset users. In a prospective longitudinal birth cohort study following 1,037 individuals (Meier et al., 2012), a significant decrease in IQ and neuropsychological performance was observed in adolescent-onset users and persisted even after abstinence from marijuana for at least one year. However, Meier et al. (2012) reported in there was no significant change in IQ in adult-onset users.
The HHS noted that there is some evidence that the severity of the persistent neurological impairments may also be due in part to the amount of marijuana usage. In the study mentioned above, Gruber et al. (2012) found that the early onset users consumed three times as much marijuana per week and used it twice as often as late onset users. Meier et al. (2012) reported in their study, mentioned above, that there was a correlation between IQ deficits in adolescent onset users and the increased amount of marijuana used.
Behavioral Effects of Prenatal Exposure
In studies that examined effects of prenatal marijuana exposure, many of the pregnant women also used alcohol and tobacco in addition to marijuana. Even though other drugs were used in conjunction with marijuana, there is evidence of an association between heavy prenatal marijuana exposure and deficits in some cognitive function. There have been two prospective longitudinal birth cohort studies following individuals prenatally exposed to marijuana from birth until adulthood: The Ottawa Prenatal Prospective Study (OPPS; Fried et al., 1980), and the Maternal Health Practices and Child Development Project (MHPCD; Day et al., 1985). Both longitudinal studies report that heavy prenatal marijuana use is associated with decreased performance on tasks assessing memory, verbal and quantitative reasoning in 4-year-olds (Fried and Watkinson, 1990) and in 6 year olds (Goldschmidt et al., 2008). In subsequent studies with the OPPS cohort, deficits in sustained attention were reported in children ages 6 and 13-16 years (Fried et al., 1992; Fried, 2002) and deficits in executive neurological function were observed in 9- and 12-year-old children (Fried et al., 1998). DEA further notes that with the MHPCD cohort, follow-up studies reported an increased rate of delinquent behavior (Day et al., 2011) and decreased achievement test scores (Goldschmidt et al., 2012) at age 14. When the MHPCD cohort was followed to age 22, there was a marginal (p = 0.06) increase in psychosis with prenatal marijuana exposure and early onset of marijuana use (Day et al., 2015).
Association of Marijuana Use With Psychosis
There has been extensive research to determine whether marijuana usage is associated with development of schizophrenia or other psychoses, and the HHS indicated that the available data do not suggest a causative link between marijuana and the development of psychosis (HHS, 2015; Minozzi et al., 2010). As mentioned in the HHS review (HHS, 2015), numerous large scale longitudinal studies demonstrated that subjects who used marijuana do not have a greater incidence of psychotic diagnoses compared to non-marijuana users (van Os et al., 2002; Fergusson et al., 2005; Kuepper et al., 2011). Further, the HHS commented that when analyzing the available data examining the association between marijuana and psychosis, it is critical to differentiate whether the patients in a study are already diagnosed with psychosis or if the individuals have a limited number of symptoms associated with psychosis without qualifying for a diagnosis of the disorder.
As mentioned by the HHS, some of the studies examining the association between marijuana and psychosis utilized non-standard methods to categorize psychosis and these methods did not conform to the criteria in the Diagnostic and Statistical Manual (DSM-5) or the International Classification of Diseases (ICD-10) and would not be appropriate for use in evaluating the association between marijuana use and psychosis. For example, researchers characterized psychosis as "schizophrenic cluster" (Maremmani et al., 2004), "subclinical psychotic symptoms" (van Gastel et al., 2012), "pre-psychotic clinical high risk" (van der Meer et al., 2012), and symptoms related to "psychosis vulnerability" (Griffith-Lendering et al., 2012).
The HHS discussed an early epidemiological study conducted by Andreasson et al. (1987), which examined the link between psychosis and marijuana use. In this study, about 45,000 18- and 19-year-old male Swedish subjects provided detailed information on their drug-taking history and 274 of these subjects were diagnosed with schizophrenia over a 14-year period (1969-1983). Out of the 274 subjects diagnosed with psychosis, 21 individuals (7.7%) had used marijuana more than 50 times, while 197 individuals (72%) never used marijuana. As presented by the authors (Andreasson et al., 1987), individuals who claimed to take marijuana on more than 50 occasions were 6 times more likely to be diagnosed with schizophrenia than those who had never consumed the drug. The authors concluded that marijuana users who are vulnerable to developing psychoses are at the greatest risk for schizophrenia. In a 35 year follow up to the subjects evaluated in Andreasson et al. (1987), Manrique-Garcia et al. (2012) reported similar findings. In the follow up study, 354 individuals developed schizophrenia. Of those, 32 individuals (9%) had used marijuana more than 50 times and were 6.3 times more likely to develop schizophrenia. 255 of the 354 individuals (72%) never used marijuana.
The HHS also noted that many studies support the assertion that psychosis from marijuana usage may manifest only in individuals already predisposed to development of psychotic disorders. Marijuana use may precede diagnosis of psychosis (Schimmelmann et al., 2011), but most reports indicate that prodromal symptoms of schizophrenia are observed prior to marijuana use (Schiffman et al., 2005). In a review examining gene-environmental interaction between marijuana exposure and the development of psychosis, it was concluded that there is some evidence to support that marijuana use may influence the development of psychosis but only for susceptible individuals (Pelayo-Teran et al., 2012). Start Printed Page 53751
Degenhardt et al. (2003) modeled the prevalence of schizophrenia against marijuana use across eight birth cohorts in individuals born during 1940 to 1979 in Australia. Even though there was an increase in marijuana use in the adult subjects over this time period, there was not an increase in diagnoses of psychosis for these same subjects. The authors concluded that use of marijuana may increase schizophrenia only in persons vulnerable to developing psychosis.
Cardiovascular and Autonomic Effects
The HHS stated that acute use of marijuana causes an increase in heart rate (tachycardia) and may increase blood pressure (Capriotti et al., 1988; Benowitz and Jones, 1975). There is some evidence that associates the increased heart rate from Δ9-THC exposure with excitation of the sympathetic and depression of the parasympathetic nervous systems (Malinowska et al., 2012). Tolerance to tachycardia develops with chronic exposure to marijuana (Jones, 2002; Sidney, 2002).
Prolonged exposure to Δ9-THC results in a decrease in heart rate (bradycardia) and hypotension (Benowitz and Jones, 1975). These effects are thought to be mediated through peripherally located, presynaptic CB1 receptor inhibition of norepinephrine release with possible direct activation of vascular cannabinoid receptors (Wagner et al., 1998; Pacher et al., 2006).
As stated in the HHS recommendation (HHS, 2015), marijuana exposure causes orthostatic hypotension (fainting-like feeling; sudden drop in blood pressure upon standing up) and tolerance can develop to this effect upon repeated, chronic exposure (Jones, 2002). Tolerance to orthostatic hypotension is potentially related to plasma volume expansion, but tolerance does not develop to supine hypotensive effects (Benowitz and Jones, 1975).
Marijuana smoking, particularly by those with some degree of coronary artery or cerebrovascular disease, poses risks such as increased cardiac work, increased catecholamines and carboxyhemoglobin, myocardial infarction and postural hypotension (Benowitz and Jones, 1981; Hollister, 1988; Mittleman et al., 2001; Malinowska et al., 2012). However, electrocardiographic changes were minimal after administration of large cumulative doses of Δ9-THC (Benowitz and Jones, 1975).
The DEA notes two recent reports that reviewed several case studies on marijuana and cardiovascular complications (Panayiotides, 2015; Hackam, 2015). Panayiotides (2015) reported that approximately 25.6% of the cardiovascular cases from marijuana use resulted in death from data provided by the French Addictovigilance Network during the period of 2006-2010. Several case studies on marijuana usage and cardiovascular events were discussed and it was concluded that although a causal link cannot be established due to not knowing exact amounts of marijuana used in the cases and confounding variables, the available evidence supports a link between marijuana and cardiotoxicity. Hackham (2015) reviewed 34 case reports or case series reports of marijuana and stroke/ischemia in 64 stroke patients and reported that in 81% of the cases there was a temporal relationship between marijuana usage and stroke or ischemic event. The author concluded that collective analysis of the case reports supports a causal link between marijuana use and stroke.
Respiratory Effects
The HHS stated that transient bronchodilation is the most typical respiratory effect of acute exposure to marijuana (Gong et al., 1984). In a recent longitudinal study, information on marijuana use and pulmonary data function were collected from 5,115 individuals over 20 years from 4 communities in the United States (Oakland, CA; Chicago, IL; Minneapolis, MN; Birmingham, AL) (Pletcher et al., 2012). Of the 5,115 individuals, 795 individuals reported use of only marijuana (without tobacco). The authors reported that occasional use of marijuana (7 joint-years for lifetime or 1 joint/day for 7 years or 1 joint/week for 49 years) does not adversely affect pulmonary function. Pletcher et al. (2012) further concluded that there is some preliminary evidence suggesting that heavy marijuana use may have a detrimental effect on pulmonary function, but the sample size of heavy marijuana users in the study was too small. Further, as mentioned in the HHS recommendation document (HHS, 2015), long-term use of marijuana may lead to chronic cough, increased sputum, as well as increased frequency of chronic bronchitis and pharyngitis (Adams and Martin, 1996; Hollister, 1986).
The HHS stated that the evidence that marijuana may lead to cancer of the respiratory system is inconsistent, with some studies suggesting a positive correlation while others do not (Lee and Hancox, 2011; Tashkin, 2005). The HHS noted a case series that reported lung cancer occurrences in three marijuana smokers (age range 31-37 years) with no history of tobacco smoking (Fung et al., 1999). Furthermore, in a case-control study (n = 173 individuals with squamous cell carcinoma of the head and neck; n = 176 controls; Zhang et al., 1999), prevalence of marijuana use was 9.7% in controls and 13.9% in cases and the authors reported that marijuana use may dose-dependently interact with mutagenic sensitivity, cigarette smoking, and alcohol use to increase risk associated with head and neck cancers (Zhang et al., 1999). However, in a large clinical study with 1,650 subjects, no positive correlation was found between marijuana use and lung cancer (Tashkin et al., 2006). This finding held true regardless of the extent of marijuana use when both tobacco use and other potential confounding factors were controlled. The HHS concluded that new evidence suggests that the effects of smoking marijuana on respiratory function and cancer are different from the effects of smoking tobacco (Lee and Hancox, 2011).
The DEA further notes the publication of recent review articles critically evaluating the association between marijuana and lung cancer. Most of the reviews agree that the association is weak or inconsistent (Huang et al., 2015; Zhang et al., 2015; Gates et al., 2014; Hall and Degenhardt, 2014). Huang et al. (2015) identified and reviewed six studies evaluating the association between marijuana use and lung cancer and the authors concluded that an association is not supported most likely due to the small amounts of marijuana smoked in comparison to tobacco. Zhang et al. (2015) examined six case control studies from the US, UK, New Zealand, and Canada within the International Lung Cancer Consortium and found that there was a weak association between smoking marijuana and lung cancer in individuals who never smoked tobacco, but precision of the association was low at high marijuana exposure levels. Hall and Degenhardt (2014) noted that even though marijuana smoke contains several of the same carcinogens and co-carcinogens as tobacco smoke (Roth et al., 1998) and has been found to be mutagenic and carcinogenic in the mouse skin test, epidemiological studies have been inconsistent, but more consistent positive associations have been reported in case control studies. Finally Gates et al. (2014), reviewed the studies evaluating marijuana use and lung cancer and concluded that there is evidence that marijuana produces changes in the respiratory system (precursors to cancer) that could lead to Start Printed Page 53752 lung cancer, but overall association is weak between marijuana use and lung cancer especially when controlling for tobacco use.
Endocrine System
Reproductive Hormones
The HHS stated that administration of marijuana to humans does not consistently alter the endocrine system. In a controlled human exposure study (n = 4 males), subjects were acutely administered smoked marijuana containing 2.8% Δ9-THC or placebo and an immediate significant decrease in luteinizing hormone and an increase in cortisol was reported in the subjects that smoked marijuana (Cone et al., 1986). Furthermore, as cited by the HHS, two later studies (Dax et al., 1989; Block et al., 1991) reported no changes in hormone levels. Dax et al. (1989) recruited male volunteers (n = 17) that were occasional or heavy users of marijuana. Following exposure to smoked Δ9-THC (18 mg/cigarette) or oral Δ9-THC (10 mg three times per day for three days and on the morning of the fourth day), the subjects in that study showed no changes in plasma adrenocorticotropic hormone (ACTH), cortisol, prolactin, luteinizing hormone, or testosterone levels. Additionally, Block et al. (1991) compared plasma hormone levels amongst non-users as well as infrequent, moderate, and frequent users of marijuana (n = 93 men and 56 women) and found that chronic use of marijuana (infrequent, moderate, and frequent users) did not significantly alter concentrations of testosterone, luteinizing hormone, follicle stimulating hormone, prolactin, or cortisol.
The HHS noted that there is a discrepancy in the effect of marijuana on female reproductive system functionality between animals and humans (HHS, 2015). Female rhesus monkeys that were administered 2.5 mg/kg Δ9-THC, i.m., during days 1-18 of the menstrual cycle had reduced progesterone levels and ovulation was suppressed (Asch et al., 1981). However, women who smoked marijuana (1 gram marijuana cigarette with 1.8% Δ9-THC) during the periovulatory period (24-36 hours prior to ovulation) did not exhibit changes in reproductive hormone levels or their menstrual cycles (Mendelson and Mello, 1984). In a review article by Brown and Dobs (2002), the authors state that endocrine changes observed with marijuana are no longer observed with chronic administration and this may be due to drug tolerance.
Reproductive Cancers
The HHS stated that recent studies support a possible association between frequent, long-term marijuana use and increased risk of testicular germ cell tumors. In a hospital-based case-control study, the frequency of marijuana use was compared between testicular germ cell tumor (TGCT) patients (n = 187) and controls (n = 148) (Trabert et al., 2011).
TGCT patients were more likely to be frequent marijuana users than controls with an odds ratio (OR) of 2.2 (95% confidence limits of 1.0-5.1) and were less likely to be infrequent or short-term users with odds ratios of 0.5 and 0.6, respectively in comparison to controls (Trabert et al., 2011). The DEA further notes that in two population-based case-control studies (Daling et al., 2009; Lacson et al., 2012), marijuana use was compared between patients diagnosed with TGCT and matched controls in Washington State or Los Angeles County. In both studies, it was reported that TCGT patients were twice as likely as controls to use marijuana. Authors of both studies concluded that marijuana use is associated with an elevated risk of TGCT (Daling et al., 2009; Lacson et al., 2012).
The HHS cited a study (Sarfaraz et al., 2005) demonstrating that WIN 55,212-2 (a mixed CB1/CB2 agonist) induces apoptosis (one form of cell death) in prostate cancer cells and decreases expression of androgen receptors and prostate specific antigens, suggesting a potential therapeutic value for cannabinoid agonists in the treatment of prostate cancer, an androgen-stimulated type of carcinoma.
Other Hormones (e.g. Thyroid, Appetite)
In more recent studies, as cited by the HHS, chronic marijuana use by subjects (n = 39) characterized as dependent on marijuana according to the ICD-10 criteria did not affect serum levels of thyroid hormones: TSH (thyrotropin), T4 (thyroxine), and T3 (triiodothyronine) (Bonnet, 2013). With respect to appetite hormones, in a pilot study with HIV-positive males, smoking marijuana dose-dependently increased plasma levels of ghrelin and leptin and decreased plasma levels of peptide YY (Riggs et al., 2012).
The HHS stated that Δ9-THC reduces binding of the corticosteroid dexamethasone in hippocampal tissue from adrenalectomized rats and acute Δ9-THC releases corticosterone, with tolerance developing to this effect with chronic administration (Eldridge et al., 1991). These data suggest that Δ9-THC may interact with the glucocorticoid receptor system.
Immune System
The HHS stated that cannabinoids alter immune function but that there can be differences between the effects of synthetic, natural, and endogenous cannabinoids (Croxford and Yamamura, 2005; Tanasescu and Constantinescu, 2010).
The HHS noted that there are conflicting results in animal and human studies with respect to cannabinoid effects on immune functioning in subjects with compromised immune systems. Abrams et al. (2003) examined the effects of marijuana and Δ9-THC in 62 HIV-1-infected patients. Subjects received one of three treatments, three times a day: smoked marijuana cigarette containing 3.95% Δ9-THC, oral tablet containing Δ9-THC (2.5 mg oral dronabinol), or oral placebo. There were no changes in CD4+ and CD8+ cell counts, HIV RNA levels, or protease inhibitor levels in any of the treatment groups (Abrams et al., 2003). Therefore, use of cannabinoids showed no short-term adverse virologic effects in individuals with compromised immune systems. Conversely, Roth et al. (2005) reported that in immunodeficient mice implanted with human blood cells infected with HIV, exposure to Δ9-THC in vivo suppresses immune function, increases HIV co-receptor expression, and acts as a cofactor to enhance HIV replication.
The DEA notes two recent clinical studies reporting a decrease in cytokine and interleukin levels following marijuana use. Keen et al. (2014) compared the differences in the levels of IL-6 (interleukin-6), a proinflammatory cytokine, amongst non-drug users (n = 78), marijuana only users (n = 46) and marijuana plus other drug users (n = 45) in a community-based sample of middle-aged African Americans (Keen et al., 2014). After adjusting for confounders, analyses revealed that lifetime marijuana only users had significantly lower IL-6 levels than the nonuser group. Further, Sexton et al. (2014) compared several immune parameters in healthy individuals and subjects with multiple sclerosis (MS) and found that the chronic use of marijuana resulted in reduced monocyte migration, and decreased levels of CCL2 and IL-17 in both healthy and MS groups.
The DEA also notes a review suggesting that Δ9-THC suppresses the immune responses in experimental animal models and in vitro and that these changes may be primarily Start Printed Page 53753 mediated through the CB2 cannabinoid receptor (Eisenstein and Meissler, 2015).
Petitioners' Major Comments in Relation to Factor 2 and the Government's Responses
(1) The petitioners state that "[m]edical use of cannabis is considered safe." (Exhibit B, page 7); and that "[t]here are adequate and well-controlled studies proving the medical efficacy of cannabis." (Exhibit B, page 10). The petitioners also allege that "Cannabis is safer than current, legal Schedule II opiate drugs" and that it presents milder side effects (Exhibit B, page 9-10).
As detailed in the HHS review and as discussed later in this document (see Factor 3), there are neither adequate safety studies nor adequate, well-controlled studies proving marijuana's efficacy. The DEA notes that neither the CSA nor established scheduling criteria suggest that the HHS and DEA should consider the relative safety profiles of drugs when determining the proper schedule. To the extent that the petitioners were referring to abuse and dependence liability, this document discusses those effects in factors 1, 4, and 7.
(2) The petitioners state that "scientific evidence regarding the safety and efficacy of cannabis is readily available directly from the National Library of Medicine." (Exhibit B, page 14).
The government agrees that many articles discuss marijuana and its constituents. Yet, these articles in no way demonstrate that marijuana is safe and effective for the treatment of any disease or condition. As mentioned in the HHS review and as discussed later in this document (see Factor 3), the current research does not provide adequate detailed scientific evidence regarding chemistry, pharmacology, toxicology, and effectiveness derived from well-controlled clinical investigations to permit a conclusion that marijuana is safe and effective for treating a specific, recognized disorder.
(3) The petitioners mentioned on page 9 of exhibit B that "[t]here has never been a lethal overdose of marijuana reported in humans" and that "[t]here is no known LD50 for any form of cannabis."
As more fully discussed in Factor 3 below, the HHS and DEA conclude that there are not adequate studies to determine the safety of marijuana. As discussed in the HHS document and below, the determination of safety is more complex than a mere determination of the rate or likelihood of death. Moreover, the lack of overdose deaths attributed to a drug is not evidence that the drug is safe for medical use.
Factor 3: The State of the Current Scientific Knowledge Regarding the Drug or Substance
Chemistry
The HHS stated that marijuana, also known as Cannabis sativa L., is part of the Cannabaceae plant family and is one of the oldest cultivated crops. The term "marijuana" is generally used to refer to a mixture of the dried flowering tops and leaves from Cannabis. Marijuana users primarily smoke the marijuana leaves, but individuals also ingest marijuana through food infused with marijuana and its extracts. Cannabis sativa is the primary species of Cannabis that is illegally marketed in the United States. Marijuana is one of three major derivatives sold as separate illicit products, the other two being hashish and hash oil. Hashish is composed of the dried and compressed cannabinoid-rich resinous material of Cannabis and is found as balls and cakes as well as other forms. Individuals may break off pieces and place them into a pipe to smoke. Hash oil, a viscous brown or amber colored liquid, is produced by solvent extraction of cannabinoids from Cannabis and contains approximately 50% cannabinoids. One to two drops of hash oil on a cigarette has been reported to produce the equivalent of a single marijuana cigarette (DEA, 2015).
The HHS indicated in its evaluation that the petitioners defined marijuana as including all Cannabis cultivated strains. However, different marijuana samples are derived from numerous cultivated strains and may have different chemical compositions including levels of Δ9-THC and other cannabinoids (Appendino et al., 2011). A consequence of having different chemical compositions in the various marijuana samples is that there will be significant differences in safety, biological, pharmacological, and toxicological profiles and therefore, according to the HHS, all Cannabis strains cannot be considered collectively because of the variations in chemical composition. Furthermore, the concentration of Δ9-THC and other cannabinoids present in marijuana may vary due to growing conditions and processing of the plant after harvesting. For example, the plant parts collected such as flowers, leaves and stems can influence marijuana's potency, quality, and purity (Adams and Martin, 1996; Agurell et al., 1984; Mechoulam, 1973). Variations in marijuana harvesting have resulted in potencies ranging from a low of 1 to 2% up to a high of 17% as indicated by cannabinoid content. The concentration of Δ9-THC averages approximately 12% by weight in a typical marijuana mixture of leaves and stems. However, some specifically grown and selected marijuana samples can contain 15% or greater Δ9-THC (Appendino et al., 2011). As a result, the Δ9-THC content in a 1 gram marijuana cigarette can range from as little as 3 milligrams to 150 milligrams or more. In a systematic review conducted by Cascini et al. (2012), it was reported that marijuana's Δ9-THC content has increased significantly from 1979-2009.
Since there is considerable variability in the cannabinoid concentrations and chemical constituency among marijuana samples, the interpretation of clinical data with marijuana is complicated. A primary issue is the lack of consistent concentrations of Δ9-THC and other substances in marijuana which complicates the interpretation of the effects of different marijuana constituents. An added issue is that the non-cannabinoid components in marijuana may potentially modify the overall pharmacological and toxicological properties of various marijuana strains and products.
Various Cannabis strains contain more than 525 identified natural constituents including cannabinoids, 21 (or 22) carbon terpenoids found in the plant, as well as their carboxylic acids, analogues, and transformation products (Agurell et al., 1984; 1986; Mechoulam, 1973; Appendino et al., 2011). To date, more than 100 cannabinoids have been characterized (ElSohly and Slade, 2005; Radwan et al., 2009; Appendino et al., 2011), and most major cannabinoid compounds occurring naturally have been identified. There are still new and comparably more minor cannabinoids being characterized (Pollastro et al., 2011). The majority of the cannabinoids are found in Cannabis. One study reported accumulation of two cannabinoids, cannabigerol and its corresponding acid, in Helichrysum (H. umbraculigerum) which is a non-Cannabis source (Appendino et al., 2011).
Of the cannabinoids found in marijuana, Δ9-THC (previously known as Δ1-THC) and delta-8-tetrahydrocannabinol (Δ8-THC, Δ6-THC) have been demonstrated to produce marijuana's psychoactive effects. Psychoactive effects from marijuana usage have been mainly attributed to Δ9-THC because Δ9-THC is present in significantly more quantities than Δ8-THC in most marijuana varieties. There are only a few marijuana strains that Start Printed Page 53754 contain Δ8-THC in significant amounts (Hively et al., 1966). Δ9-THC is an optically active resinous substance that is extremely lipophilic. The chemical name for Δ9-THC is (6a R-trans)-6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-6 H-dibenzo-[b,d]pyran-1-ol, or (-)-delta9-(trans)-tetrahydrocannabinol. The (-)-trans Δ9-THC isomer is pharmacologically 6 to 100 times more potent than the (+)-trans isomer (Dewey et al., 1984).
Other relatively well-characterized cannabinoids present in marijuana include cannabidiol (CBD), cannabichromene (CBC), and cannabinol (CBN). CBD and CBC are major cannabinoids in marijuana and are both lipophilic. The chemical name for CBD is 2-[(1 R, 6 R)-3-methyl-6-prop-1-en-2-ylcyclohex-2-en-1-yl]-5-pentylbenzene-1,3-diol and the chemical name for CBC is 2-methyl-2-(4-methylpent-3-enyl)-7-pentyl-5-chromenol. CBN is a minor naturally-occurring cannabinoid with weak psychoactivity and is also a major metabolite of Δ9-THC. The chemical name for CBN is 6,6,9-trimethyl-3-pentyl-benzo[c]chromen-1-ol.
In summary, marijuana has several strains with high variability in the concentrations of Δ[9] -THC, the main psychoactive component, as well as other cannabinoids and compounds. Marijuana is not a single chemical and does not have a consistent and reproducible chemical profile with predictable or consistent clinical effects. In the HHS recommendation for marijuana scheduling (HHS, 2015), it was recommended that investigators consult a guidance for industry entitled, Botanical Drug Products, [46] which provides information on the approval of botanical drug products. Specifically, in order to investigate marijuana in support of a New Drug Application (NDA), clinical studies under an Investigational New Drug (IND) application should include "consistent batches of a particular marijuana product for [a] particular disease." (HHS, 2015). Furthermore, the HHS noted that investigators must provide data meeting the requirements for new drug approval as stipulated in 21 CFR 314.50 (HHS, 2015).
Human Pharmacokinetics
Pharmacokinetics of marijuana in humans is dependent on the route of administration and formulation (Adams and Martin, 1996; Agurell et al., 1984; Agurell et al., 1986). Individuals primarily smoke marijuana as a cigarette (weighing between 0.5 and 1 gram) or in a pipe. More recently, vaporizers have been used as another means for individuals to inhale marijuana. Marijuana may also be ingested orally in foods or as an extract in ethanol or other solvents. Pharmacokinetic studies with marijuana focused on evaluating the absorption, metabolism, and elimination profile of Δ9-THC and other cannabinoids (Adams and Martin, 1996; Agurell et al., 1984; Agurell et al., 1986).
Absorption and Distribution of Inhaled Marijuana Smoke
There is high variability in the pharmacokinetics of Δ9-THC and other cannabinoids from smoked marijuana due to differences in individual smoking behavior even under controlled experimental conditions (Agurell et al., 1986; Herning et al., 1986; Huestis et al., 1992a). Experienced marijuana users can titrate and regulate the dose by holding marijuana smoke in their lungs for an extended period of time resulting in increased psychoactive effects by prolonging absorption of the smoke. This property may also help explain why there is a poor correlation between venous levels of Δ9-THC and the intensity of effects and intoxication (Agurell et al., 1986; Barnett et al., 1985; Huestis et al., 1992a). The HHS recommended that puff and inhalation volumes should be tracked in experimental studies because the concentration of cannabinoids can vary at different stages of smoking.
Δ9-THC from smoked marijuana is rapidly absorbed within seconds. Psychoactive effects are observed immediately following absorption with measurable neurological and behavioral changes for up to 6 hours (Grotenhermen, 2003; Hollister, 1986; Hollister, 1988). Δ9-THC is distributed to the brain in a rapid and efficient manner. Bioavailability of Δ9-THC from marijuana (from a cigarette or pipe) ranges from 1 to 24% with the fraction absorbed rarely exceeding 10 to 20% (Agurell et al., 1986; Hollister, 1988). The low and variable bioavailability of Δ9-THC is due to loss in side-stream smoke, variation in individual smoking behaviors and experience, incomplete absorption of inhaled smoke, and metabolism in lungs (Herning et al., 1986; Johansson et al., 1989). After cessation of smoking, Δ9-THC venous levels decline within minutes and continue to decline to about 5% to 10% of the peak level within an hour (Agurell et al., 1986; Huestis et al., 1992a; Huestis et al., 1992b).
Absorption and Distribution of Orally Administered Marijuana
Following oral administration of Δ9-THC or marijuana, onset of effects start within 30 to 90 minutes, peak after 2 to 3 hours and effects remain for 4 to 12 hours (Grotenhermen, 2003; Adams and Martin, 1996; Agurell et al., 1984; Agurell et al., 1986). Dose titration of Δ9-THC from orally ingested marijuana is difficult for users in comparison to smoked or inhaled marijuana due to the delay in the onset of effects. Oral bioavailability of Δ9-THC, either in its pure form or in marijuana, is low and variable with a range from 5% to 20% (Agurell et al., 1984; Agurell et al., 1986). There is also inter- and intra-subject variability of orally administered Δ9-THC under experimental conditions and even under repeated dosing experiments (HHS, 2015). The HHS noted that in bioavailability studies using radiolabeled Δ9-THC, Δ9-THC plasma levels following oral administration of Δ9-THC were low relative to plasma levels after inhaled or intravenously administered Δ9-THC. The low and variable bioavailability of orally administered Δ9-THC is due to first pass hepatic elimination from blood and erratic absorption from stomach and bowel (HHS, 2015).
Metabolism and Excretion of Cannabinoids From Marijuana
Studies evaluating cannabinoid metabolism and excretion focused on Δ9-THC because it is the primary psychoactive component in marijuana. Δ9-THC is metabolized via microsomal hydroxylation and oxidation to both active and inactive metabolites (Lemberger et al., 1970; Lemberger et al., 1972a; Lemberger et al., 1972b; Agurell et al., 1986; Hollister, 1988). Metabolism of Δ9-THC is consistent among frequent and infrequent marijuana users (Agurell et al., 1986). The primary active metabolite of Δ9-THC following oral ingestion is 11-hydroxy-Δ9-THC which is equipotent to Δ9-THC in producing marijuana-like subjective effects (Agurell et al., 1986; Lemberger and Rubin, 1975). Metabolite levels following oral administration may be greater than that of Δ9-THC and may contribute greatly to the pharmacological effects of oral Δ9-THC or marijuana.
Plasma clearance of Δ9-THC approximates hepatic blood flow at a rate of approximately 950 ml/min or greater. Rapid clearance of Δ9-THC from blood is primarily due to redistribution to other tissues in the body rather than to metabolism (Agurell et al., 1984; Agurell et al., 1986). Outside of the Start Printed Page 53755 liver, metabolism in most tissues is considerably slow or does not occur. The elimination half-life of Δ9-THC ranges from 20 hours to between 10 and 13 days (Hunt and Jones, 1980). Lemberger et al. (1970) reported that the half-life of Δ9-THC ranged from 23-28 hours in heavy marijuana users and up to 60 to 70 hours in naïve users. The long elimination half-life of Δ9-THC is due to slow release of Δ9-THC and other cannabinoids from tissues and subsequent metabolism. Inactive carboxy metabolites of Δ9-THC have terminal half-lives of 50 hours to 6 days or more and serve as long-term markers in urine tests for marijuana use.
Most of the absorbed Δ9-THC dose is eliminated in the feces and about 33% in urine. The glucuronide metabolite of Δ9-THC is excreted as the major urine metabolite along with 18 non-conjugated metabolites (Agurell et al., 1986).
Research Status and Test of Currently Accepted Medical Use for Marijuana
According to the HHS, there are numerous human clinical studies with marijuana in the United States under FDA-regulated IND applications. Results of small clinical exploratory studies have been published in the medical literature. Approval of a human drug for marketing, however, is contingent upon FDA approval of a New Drug Application (NDA) or a Biologics License Application (BLA). According to the HHS, the FDA has not approved any drug product containing marijuana for marketing.
The HHS noted that a drug may be found to have a medical use in treatment in the United States for purposes of the CSA if the drug meets the five elements described by the DEA in 1992. Those five elements "are both necessary and sufficient to establish a prima facie case of currently accepted medical use" in treatment in the United States." (57 FR 10499, 10504 (March 26, 1992)). This five-element test, which the HHS and DEA have utilized in all such analyses for more than two decades, has been upheld by the Court of Appeals. ACT, 15 F.3d at 1135. The five elements that characterize "currently accepted medical use" for a drug are summarized here and expanded upon in the discussion below:
1. The drug's chemistry must be known and reproducible;
2. There must be adequate safety studies;
3. There must be adequate and well-controlled studies proving efficacy;
4. The drug must be accepted by qualified experts; and
5. Scientific evidence must be widely available.
In its review (HHS, 2015), the HHS evaluated the five elements with respect to the currently available research for marijuana. The HHS concluded that marijuana does not meet any of the five elements—all of which must be demonstrated to find that a drug has a "currently accepted medical use." A brief summary of the HHS's evaluation is provided below.
Element #1: The drug's chemistry must be known and reproducible.
"The substance's chemistry must be scientifically established to permit it to be reproduced into dosages which can be standardized. The listing of the substance in a current edition of one of the official compendia, as defined by section 201(j) of the Food, Drug and Cosmetic Act, 21 U.S.C. 321(j), is sufficient generally to meet this requirement." 57 FR 10499, 10506 (March 26, 1992).
Marijuana, as defined in the petition, includes all Cannabis strains. (For purposes of the CSA, marijuana includes all species of the genus Cannabis, including all strains therein[47] ). Based on the definition of marijuana in the petition, the chemistry of marijuana is not reproducible such that a standardized dose can be created. Chemical constituents including Δ9-THC and other cannabinoids vary significantly in marijuana samples derived from different strains (Appendino et al., 2011). As a result, there will be significant differences in safety, biological, pharmacological, and toxicological parameters amongst the various marijuana samples. Due to the variation of the chemical composition in marijuana samples, it is not possible to reproduce a standardized dose when considering all strains together. The HHS does advise that if a specific Cannabis strain is cultivated and processed under controlled conditions, the plant chemistry may be consistent enough to derive reproducible and standardized doses.
Element #2: There must be adequate safety studies.
"There must be adequate pharmacological and toxicological studies, done by all methods reasonably applicable, on the basis of which it could fairly and responsibly be concluded, by experts qualified by scientific training and experience to evaluate the safety and effectiveness of drugs, that the substance is safe for treating a specific, recognized disorder." 57 FR 10499, 10506 (March 26, 1992).
The HHS stated that there are no adequate safety studies on marijuana. As indicated in their evaluation of Element #1, the considerable variation in the chemistry of marijuana complicates the safety evaluation. The HHS concluded that marijuana does not satisfy Element #2 for having adequate safety studies such that medical and scientific experts may conclude that it is safe for treating a specific ailment.
Element #3: There must be adequate and well-controlled studies of efficacy.
"There must be adequate, well-controlled, well-designed, well-conducted and well-documented studies, including clinical investigations, by experts qualified by scientific training and experience to evaluate the safety and effectiveness of drugs, on the basis of which it could be fairly and responsibly concluded by such experts that the substance will have the intended effect in treating a specific, recognized disorder." 57 FR 10499, 10506 (March 26, 1992).
As indicated in the HHS's review of marijuana (HHS, 2015), there are no adequate or well-controlled studies that prove marijuana's efficacy. The FDA independently reviewed (FDA, 2015) publicly available clinical studies on marijuana published prior to February 2013 to determine if there were appropriate studies to determine marijuana's efficacy (please refer to FDA, 2015 and HHS, 2015 for more details). After review, the FDA determined that out of the identified articles, including those identified through a search of bibliographic references and 566 abstracts located on PubMed, 11 studies met the a priori selection criteria, including placebo control and double-blinding. FDA and HHS critically reviewed each of the 11 studies to determine if the studies met accepted scientific standards. FDA and HHS concluded that these studies do not "currently prove efficacy of marijuana" for any therapeutic indication due to limitations in the study designs. The HHS indicated that these studies could be used as proof of Start Printed Page 53756 concept studies, providing preliminary evidence on a proposed hypothesis involving a drug's effect.
Element #4: The drug must be accepted by qualified experts.
"[A] consensus of the national community of experts, qualified by scientific training and experience to evaluate the safety and effectiveness of drugs, accepts the safety and effectiveness of the substance for use in treating a specific, recognized disorder. A material conflict of opinion among experts precludes a finding of consensus." 57 FR 10499, 10506 (March 26, 1992).
The HHS concluded that there is currently no evidence of a consensus among qualified experts that marijuana is safe and effective in treating a specific and recognized disorder. The HHS indicated that medical practitioners who are not experts in evaluating drugs cannot be considered qualified experts (HHS, 2015; 57 FR 10499, 10505). Further, the HHS noted that the 2009 American Medical Association (AMA) report entitled, "Use of Cannabis for Medicinal Purposes" does not conclude that there is a currently accepted medical use for marijuana. HHS also pointed out that state-level "medical marijuana" laws do not provide evidence of such a consensus among qualified experts.
Element #5: The scientific evidence must be widely available.
"In the absence of NDA approval, information concerning the chemistry, pharmacology, toxicology, and effectiveness of the substance must be reported, published, or otherwise widely available, in sufficient detail to permit experts, qualified by scientific training and experience to evaluate the safety and effectiveness of drugs, to fairly and responsibly conclude the substance is safe and effective for use in treating a specific, recognized disorder." 57 FR 10499, 10506 (March 26, 1992).
The HHS concluded that the currently available data and information on marijuana is not sufficient to allow scientific scrutiny of the chemistry, pharmacology, toxicology, and effectiveness. In particular, scientific evidence demonstrating the chemistry of a specific Cannabis strain that could provide standardized and reproducible doses is not available.
Petitioners' Major Comments in Relation to Factor 3 and the Government's Responses
(1) The petitioners indicate that there is medical support and acceptance for the medical use of marijuana and stated that "[c]annabis has been accepted by the medical community as meeting the current, modern accepted standards for what constitutes medicine." (Exhibit B, page 13). On page 3 of the cover letter of the petition, the petitioners stated, "The American medical community supports rescheduling, and there are safe pharmacy-based methods to dispense medical cannabis."
Furthermore, they stated that "[i]n 2009, the American Medical Association (AMA) reversed its earlier position that supported [s]chedule I classification of cannabis. The AMA now supports investigation and clinical research of cannabis for medicinal use, and urged the federal government to reassess the [s]chedule I classification. The American College of Physicians [ACP] recently expressed similar support." In addition, they note that the Institute of Medicine (IOM) also documented the scientific basis and therapeutic effects of cannabis (Exhibit B, page 13).
The DEA notes that the statements by the cited organizations (AMA, ACP, IOM) support more research into the potential medical properties associated with marijuana. The HHS did not find that the statements by these organizations provide evidence supporting a conclusion that adequate safety studies and adequate, well-controlled efficacy studies demonstrate the safety and efficacy of marijuana (HHS, 2015). The AMA's official policy on medicinal use of marijuana is as follows: "Our AMA urges that marijuana's status as a federal [s]chedule I controlled substance be reviewed with the goal of facilitating the conduct of clinical research and development of cannabinoid-based medicines, and alternative delivery methods. This should not be viewed as an endorsement of state-based medical cannabis programs, the legalization of marijuana, or that scientific evidence on the therapeutic use of cannabis meets the current standards for a prescription drug product." (AMA, 2009).
The DEA further notes that the 2013 AMA House of Delegates report states that, "cannabis is a dangerous drug and as such is a public health concern." (AMA, 2013). In 2008, the ACP indicated that "further research is needed to compare cannabinoids' efficacy and safety with current treatments." (ACP, 2008). The ACP stated that, "ACP urges an evidence-based review of marijuana's status as a [s]chedule I controlled substance to determine whether it should be reclassified to a different schedule. This review should consider the scientific findings regarding marijuana's safety and efficacy in some clinical conditions as well as evidence on the health risks associated with marijuana consumption, particularly in its crude smoked form" (ACP, 2008). The IOM, consistent with others in the medical community, endorses further studies into the potential therapeutic uses of marijuana, but did not advocate for medicinal use without further testing (IOM, 2009).
As detailed in the HHS review, in order for a drug to be found to have a "currently accepted medical use," it must be accepted by qualified experts. There is no evidence that there is a consensus among qualified experts that marijuana is safe and effective for use in treating a specific, recognized disorder.
(2) The petitioners claim that, "The chemistry of cannabis is known and reproducible" (Exhibit B, page 6) and "newer medicinal strains of cannabis are lower in THC and higher in the non-psychoactive, more therapeutic cannabinoids, such as CBD, and CBN. These compounds further improved the efficacy of cannabis." (Exhibit B, page 10).
As indicated by the HHS, the petitioners defined marijuana to include all Cannabis strains. As such, the chemistry of marijuana is not reproducible such that a standardized dose can be created. Chemical constituents including Δ9-THC and other cannabinoids vary significantly in different marijuana samples (Appendino et al., 2011). Furthermore, the HHS cited a published report that indicates that new substances in marijuana are continually being characterized (Pollastro et al., 2011). If there is significant variance in the chemical composition of marijuana between samples, it is not possible for the chemistry to be reproducible.
Because the petition defines marijuana as including all cultivated strains, the DEA believes that the THC and CBD level of specific strains is not relevant to this consideration. In fact, the average Δ9-THC content in marijuana has steadily risen from 1995 to 2014 as reported by the University of Mississippi Potency Monitoring Project, as presented in Factor 1. In 1995, the Δ9-THC content was 4% on average and by 2015, the average content of THC had risen to 11.2% over a 20 year period. In the same time period, CBD and CBN percentages have ranged from 0.15% to 0.60% on average.
The DEA also notes statements in the petitioners' document that support the conclusion reached by DEA and HHS that the chemistry of marijuana as broadly defined by the petitioners is not reproducible or well-defined. For example, the petitioners acknowledge that "Cannabis is a complex plant, with several subtypes of cannabis." (Exhibit Start Printed Page 53757 B, page 6). The petitioners also acknowledge that "the ratios of the various cannabinoids differ according to the plant strain, and, to some extent, how the plant is grown." (Exhibit B, page 12).
(3) The petitioners stated in Exhibit B, page 8, that "[o]verall, the 33 completed and published American controlled clinical trials with cannabis have studied its safety, routes of administration, and use in comparison with placebos, standard drugs, and in some cases dronabinol . . . ," and further cited a systematic review by Wang et al. (2008), that evaluated 23 randomized controlled trials and 8 observational studies, stating that, "[o]f all the adverse events reported, 97 percent were considered `not serious,' with the most commonly reported `dizziness.' "
The petitioners also cited in Exhibit B, page 8, "There has been a long-term, prospective, federally funded cannabis clinical study jointly administered by National Institute on Drug Abuse (NIDA) and FDA. This study has been running for over 30 years without any demonstrable adverse outcomes related to chronic medicinal cannabis use."
As cited in the HHS recommendation document (HHS, 2015), the FDA conducted its own evaluation of the published clinical studies on the medical application of marijuana prior to February 2013 (FDA, 2015). Further details on the FDA review can be found in the published report (FDA, 2015). Based on the analysis, 11 studies were evaluated further and the FDA concluded that none of these studies "meet the criteria required by the FDA to determine if marijuana is safe and effective in specific therapeutic areas." (page 6; FDA, 2015).
The DEA has reviewed the systematic review by Wang et al. (2008) and notes that most of the studies included in the review were synthetic cannabinoid medicines (e.g. dronabinol) or cannabinoid extracts (e.g. Sativex®); these types of studies were excluded in the FDA review as the analysis focused solely on natural forms of marijuana (FDA, 2015). Wang et al. (2008) concluded that "good safety and efficacy data on smoked cannabis are urgently needed."
With respect to the 30-year study cited by the petitioners (Russo et al., 2001) on page 8 of Exhibit B, it should be clarified that the referenced study was not jointly administered by NIDA and the FDA. As with other clinical studies, an IND application was approved by the FDA and marijuana was supplied by NIDA. The authors evaluated only 8 patients over this period, of which one patient died. While the findings cited by the petitioners and authors (e.g. no adverse outcomes with long term marijuana use) are informative, conclusions on long-term use of marijuana cannot be applied to the general population.
Factor 4: Its History and Current Pattern of Abuse
Marijuana continues to be the most widely used illicit drug. In 2013, an estimated 24.6 million Americans age 12 or older were current (past month) illicit drug users. Of those, 19.8 million were current (past month) marijuana users. As of 2013, an estimated 114.7 million Americans age 12 and older had used marijuana or hashish in their lifetime and 33.0 million had used it in the past year.
According to the NSDUH estimates, 3.0 million people age 12 or older used an illicit drug for the first time in 2014. Marijuana initiates totaled 2.6 million in 2014. Nearly half (46.8%) of the 2.6 million new users were less than 18 years of age. In 2014, marijuana was used by 82.2% of current (past month) illicit drug users. In 2014, among past year marijuana users age 12 or older, 18.5% used marijuana on 300 or more days within the previous 12 months. This translates into 6.5 million people using marijuana on a daily or almost daily basis over a 12-month period, a significant increase from the 3.1 million daily or almost daily users in 2006 and from the 5.7 million in just the previous year. In 2014, among past month marijuana users, 41.6% (9.2 million people) used the drug on 20 or more days in the past month, a significant increase from the 8.1 million in 2013.
Marijuana is also the illicit drug with the highest numbers of past year dependence or abuse in the U.S. population. According to the 2014 NSDUH report, of the 7.1 million persons aged 12 or older who were classified with illicit drug dependence or abuse, 4.2 million of them abused or were dependent on marijuana (representing 59.0% of all those classified with illicit drug dependence or abuse and 1.6% of the total U.S. non-institutionalized population aged 12 or older).
According to the 2015 Monitoring the Future (MTF) survey, marijuana is used by a large percentage of American youths, and is the most commonly used illicit drug among American youth. Among students surveyed in 2015, 15.5% of 8th graders, 31.1% of 10th graders, and 44.7% of 12th graders reported that they had used marijuana in their lifetime. In addition, 11.8%, 25.4%, and 34.9% of 8th, 10th, and 12th graders, respectively, reported using marijuana in the past year. A number of high school students reported daily use in the past month, including 1.1%, 3.0%, and 6.0% of 8th, 10th, and 12th graders, respectively.
The prevalence of marijuana use and abuse is also indicated by criminal investigations for which drug evidence was analyzed in federal, state, and local forensic laboratories, as discussed above in Factor 1. The National Forensic Laboratory System (NFLIS), a DEA program, systematically collects drug identification results and associated information from drug cases submitted to and analyzed by federal, state, and local forensic laboratories. NFLIS data shows that marijuana was the most frequently identified drug from January 2001 through December 2014. In 2014, marijuana accounted for 29.3% (432,989) of all drug exhibits in NFLIS.
The high consumption of marijuana is being fueled by increasing amounts of domestically grown marijuana as well as increased amounts of foreign source marijuana being illicitly smuggled into the United States. In 2014, the Domestic Cannabis Eradication and Suppression Program (DCE/SP) reported that 3,904,213 plants were eradicated in outdoor cannabis cultivation areas compared to 2,597,798 in 2000, as shown above in Table 3. Significant quantities of marijuana were also eradicated from indoor cultivation operations. There were 396,620 indoor plants eradicated in 2014 compared to 217,105 eradicated in 2000. As shown in Table 2 above, in 2014, the National Seizure System (NSS) reported seizures of 1,767,741 kg of marijuana.
Petitioners' Major Comments in Relation to Factor 4 and the Government's Responses
(1) The petitioners indicated that the history and current pattern of abuse is difficult to estimate since "a large percentage of United States citizens" have used marijuana at least once in their lifetime and some estimates have indicated that "over 40 percent of the nation has tried the plant." Further, the petitioners stated that "trying marijuana once should not be confused with a health problem, let alone a diagnosis of dependence or abuse." (Exhibit B, page 26).
Marijuana usage numbers mentioned in both the HHS Recommendation and this DEA document include surveys from NSDUH and MTF. These surveys measure extent of use of marijuana. As mentioned in this Factor, according to the results of the 2013 NSDUH survey, 17.4% of past year marijuana users age 12 or older used marijuana on 300 or Start Printed Page 53758 more days within the previous 12 months. This indicates that 5.7 million people used marijuana on a daily or almost daily basis over this 12-month period, which is a 1.8-fold increase from the 3.1 million daily or almost daily users in 2006. Furthermore, 6% of all twelfth graders in the United States reported daily use of marijuana in the 2015 MTF survey. These data strongly indicate that there is a significant portion of the U.S. population using marijuana on a daily basis.
(2) As stated in Exhibit B on page 26, subpart A, "Rates of dependence or abuse are remarkably low" and further suggest that "[i]nterviews for the National Longitudinal Alcohol Epidemiological Survey ([NLAES] [sic] and National Epidemiological Survey on Alcohol and Related Conditions ([NESARC] [sic] each confirm that rates of dependence or abuse of cannabis have never exceed (sic) two percent in a given year."
The authors of study cited by the petitioners (Compton et al., 2004) concluded that a higher percentage of American adults had a marijuana use disorder in 2001-2002 (1.5%) than in 1991-1992 (1.2%). Compton et al. (2004) noted that the marijuana use disorder increase of 0.3% over the 10 year period would equate to an increase from 2.2 million people to 3 million people in the United States. The petitioners failed to explain the impact of 1.5% (or less than 2 percent) of the U.S. population having a marijuana use disorder. In order to put these numbers into perspective, the DEA reviewed the literature and found that non-medical prescription drug use and abuse rates were examined in the same NLAES and NESARC (1991-1992 and 2001-2002) populations (Blanco et al., 2007). Blanco et al (2007) examined non-medical prescription drug use and abuse rates from the periods of 1991-1992 and 2001-2002. In 1991 through 1992, the prevalence of non-medical prescription drug (opioid, stimulant, and tranquilizer) abuse and dependence was 0.1%. Non-medical prescription drug (primarily opioid-based drugs) abuse and dependence increased to 0.3% in 2001 through 2002. Therefore, in the same 2001-2002 NLAES and NESARC populations, the percentage of people with a marijuana use disorder was approximately five-fold higher (1.5% versus 0.3%) than those with opioid abuse and dependence resulting from non-medical prescription drug use.
Further, Volkow et al. (2014) reported that in long-term or heavy marijuana users, 9% of users become addicted to marijuana. This percentage increases to 17% when marijuana use starts in adolescence and it increases to 25 to 50% of those who are daily users.
Factor 5: The Scope, Duration, and Significance of Abuse
Abuse of marijuana is widespread and significant. As previously noted, according to the NSDUH, in 2014, an estimated 117.2 million Americans (44.2%) age 12 or older had used marijuana or hashish in their lifetime, 35.1 million (13.2%) had used it in the past year, and 22.2 million (8.4%) had used it in the past month. Past year and past month marijuana use has increased significantly since 2013. Past month marijuana use is highest among 18-21 year olds and it declines among those 22 years of age and older. In 2014, an estimated 18.5% of past year marijuana users age 12 or older used marijuana on 300 or more days within the past 12 months. This translates into 6.5 million persons using marijuana on a daily or almost daily basis over a 12-month period. In 2014, an estimated 41.6% (9.2 million) of past month marijuana users age 12 or older used the drug on 20 or more days in the past month (SAMHSA, NSDUH). Chronic use of marijuana is associated with a number of health risks (see Factors 2 and 6).
Furthermore, the average percentage of Δ9-THC in seized marijuana has increased over the past two decades (The University of Mississippi Potency Monitoring Project). Additional studies are needed to clarify the impact of greater potency, but one study shows that higher levels of Δ9-THC in the body are associated with greater psychoactive effects (Harder and Rietbrock, 1997), which can be correlated with higher abuse potential (Chait and Burke, 1994).
TEDS data show that in 2013, marijuana/hashish was the primary substance of abuse in 16.8% of all admissions to substance abuse treatment among patients age 12 and older. TEDS data also show that marijuana/hashish was the primary substance of abuse for 77.0% of all 12- to 14-year-olds admitted for drug treatment and 75.5% of all 15- to 17-year-olds admitted for drug treatment in 2013. Among the 281,991 admissions to drug treatment in 2013 in which marijuana/hashish was the primary drug, the average age at admission was 25 years and the peak age cohort was 15 to 17 years (22.5%). Thirty-nine percent of the 281,991 primary marijuana/hashish admissions (35.9%) were under the age of 20.
In summary, the recent statistics from these various surveys and databases (see Factor 1 for more details) demonstrate that marijuana continues to be the most commonly used illicit drug, with large incidences of heavy use and dependence in teenagers and young adults.
Petitioners' Major Comment in Relation to Factor 5 and DEA's Response
(1) Petitioners' contend that, "The prevalence and significance of potential abuse are limited for cannabis, especially in relation to other [s]chedule II substances." The petitioners cited results from the 1990 NIDA Household Survey on Drug Abuse and indicated that, "more than four out of five people who had used cannabis in the previous year reported no problems related to the drug." (Exhibit B, page 28).
The prevalence of marijuana usage and marijuana dependence is significant in the United States. The 2014 NSDUH findings indicate that there are approximately 6.5 million Americans using marijuana on a daily or almost daily basis. Further, Volkow et al. (2014) reported that in long-term or heavy marijuana users, 9% of users become addicted to marijuana. Among those who began using marijuana in adolescence, marijuana dependence increases to 17%, and it further increases to 25 to 50% of daily users that started using marijuana during adolescence. These collective findings indicate that there is considerable significance associated with marijuana use and abuse since 9% of users become addicted to marijuana, 25 to 50% of daily marijuana users started during adolescence, and prevalence of usage is significantly high based on the data presented from Volkow et al (2014) and the 2014 NSDUH survey.
Factor 6: What, if any, Risk There is to the Public Health
In its recommendation, the HHS discussed public health risks associated with acute and chronic marijuana use in Factor 6. Public health risks as measured by emergency department visits and drug treatment admissions are discussed by HHS and DEA in Factors 1, 4, and 5. Similarly, Factor 2 discusses marijuana's pharmacology and presents some of the adverse health effects associated with use. Marijuana use may affect the physical and/or psychological functioning of an individual user, but may also have broader public impacts including driving impairments and fatalities from car accidents.
Risks From Acute Use of Marijuana
As discussed in the HHS review document (HHS, 2015), acute usage of marijuana impairs psychomotor performance including motor control and impulsivity, risk taking and executive function (Ramaekers et al., 2004; Ramaekers et al., 2006). In a Start Printed Page 53759 minority of individuals using marijuana, dysphoria, prolonged anxiety, and psychological distress may be observed (Haney et al., 1999). The DEA further notes a recent review of acute marijuana effects (Wilkinson et al., 2014) that reported impaired neurological function including altered perception, paranoia, delayed response time, and memory deficits.
In its recommendation, HHS references a meta-analysis conducted by Li et al (2012) where the authors concluded that psychomotor impairments associated with acute marijuana usage have also been associated with increased risk of car accidents with individuals experiencing acute marijuana intoxication (Li et al., 2012; HHS, 2015). The DEA further notes more recent studies examining the risk associated with marijuana use and driving. Younger drivers (under 21) have been characterized as the highest risk group associated with marijuana use and driving (Whitehill et al., 2014). Furthermore, in 2013, marijuana was found in 13% of the drivers involved in automobile-related fatal accidents (McCartt, 2015). The potential risk of automobile accidents associated with marijuana use appears to be increasing since there has been a steady increase in individuals intoxicated with marijuana over the past 20 years (Wilson et al., 2014). However, a recent study commissioned by the National Highway Traffic Safety Administration (NHTSA) reported that when adjusted for confounders (e.g., alcohol use, age, gender, ethnicity), there was not a significant increase in crash risk (fatal and nonfatal, n = 2,682) associated with marijuana use (Compton and Berning, 2015).
The DEA also notes recent studies examining unintentional exposures of children to marijuana (Wang et al., 2013; 2014). Wang et al. (2013) reviewed emergency department (ED) visits at a children's hospital in Colorado from January 1, 2005 to December 31, 2011. As stated by the authors, in 2000 Colorado passed Amendment 20 which allowed for the use of marijuana. Following the passage of "a new Justice Department policy" instructing "federal prosecutors not to seek arrest of medical marijuana users and suppliers as long as they conform to state laws" (as stated in Wang et al., 2013), 14 patients in Colorado under the age of 12 were admitted to the ED for the unintended use of marijuana over a 27 month period. Prior to the passage of this policy, from January 1, 2005 to September 30, 2009 (57 months), there were no pediatric ED visits due to unintentional marijuana exposure (Wang et al., 2013). The DEA also notes a larger scale evaluation of pediatric exposures using the National Poison Data System (Wang et al., 2014). That study reported that there were 985 unintentional marijuana exposures in children (9 years and younger) between January 1, 2005 to December 31, 2011. The authors stratified the ED visits by states with laws allowing medical use of marijuana, states transitioning to legalization for medical use, and states with no such laws. Out of the 985 exposures, 495 were in non-legal states (n = 33 states), 93 in transitional states (n = 8 states), and 396 in "legal" states (n = 9 states). The authors reported that there was a twofold increase (OR = 2.1) in moderate or major effects in children with unintentional marijuana use and a threefold increase (OR = 3.4) in admissions to critical care units in states allowing medical use of marijuana, in comparison to non-legal states.
Risks Associated With Chronic Use of Marijuana
The HHS noted that a major risk from chronic marijuana use is a distinctive withdrawal syndrome, as described in the 2013 DSM-5. The HHS analysis also quoted the following description of risks associated with marijuana [cannabis] abuse from the DSM-5:
Individuals with cannabis use disorder may use cannabis throughout the day over a period of months or years, and thus may spend many hours a day under the influence. Others may use less frequently, but their use causes recurrent problems related to family, school, work, or other important activities (e.g., repeated absences at work; neglect of family obligations). Periodic cannabis use and intoxication can negatively affect behavioral and cognitive functioning and thus interfere with optimal performance at work or school, or place the individual at increased physical risk when performing activities that could be physically hazardous (e.g. driving a car; playing certain sports; performing manual work activities, including operating machinery). Arguments with spouses or parents over the use of cannabis in the home, or its use in the presence of children, can adversely impact family functioning and are common features of those with cannabis use disorder. Last, individuals with cannabis use disorder may continue using marijuana despite knowledge of physical problems (e.g. chronic cough related to smoking) or psychological problems (e.g. excessive sedation or exacerbation of other mental health problems) associated with its use. (HHS 2015, page 34).
The HHS stated that chronic marijuana use produces acute and chronic adverse effects on the respiratory system, memory and learning. Regular marijuana smoking can produce a number of long-term pulmonary consequences, including chronic cough and increased sputum (Adams and Martin, 1996), and histopathologic abnormalities in bronchial epithelium (Adams and Martin, 1996).
Marijuana as a "Gateway Drug"
The HHS reviewed the clinical studies evaluating the gateway hypothesis in marijuana and found them to be limited. The primary reasons were: (1) Recruited participants were influenced by social, biological, and economic factors that contribute to extensive drug abuse (Hall and Lynskey, 2005), and (2) most studies testing the gateway drug hypothesis for marijuana use the determinative measure any use of an illicit drug rather than applying DSM-5 criteria for drug abuse or dependence (DSM-5, 2013).
The HHS cited several studies where marijuana use did not lead to other illicit drug use (Kandel and Chen, 2000; von Sydow et al., 2002; Nace et al., 1975). Two separate longitudinal studies with adolescents using marijuana did not demonstrate an association with use of other illicit drugs (Kandel and Chen, 2000; von Sydow et al., 2002).
It was noted by the HHS that, when evaluating the gateway hypothesis, differences appear when examining use versus abuse or dependence of other illicit drugs. Van Gundy and Rebellon (2010) reported that there was a correlation between marijuana use in adolescence and other illicit drug use in early adulthood, but when examined in terms of drug abuse of other illicit drugs, age-linked stressors and social roles were confounders in the association. Degenhardt et al. (2009) reported that marijuana use often precedes use of other illicit drugs, but dependence involving drugs other than marijuana frequently correlated with higher levels of illicit drug abuse. Furthermore, Degenhardt et al. (2010) reported that in countries with lower prevalence of marijuana usage, use of other illicit drugs before marijuana was often documented.
Based on these studies among others, the HHS concluded that although many individuals with a drug abuse disorder may have used marijuana as one of their first illicit drugs, this does not mean that individuals initiated with marijuana inherently will go on to become regular users of other illicit drugs. Start Printed Page 53760
Petitioners' Major Comment in Relation to Factor 6 and the Government's Responses
(1) The petitioners commented that marijuana does not significantly impact social behavior in domains such as motivation, driving, aggression, or hostility (Exhibit B, pages 30-41).
The HHS concluded that "Marijuana's acute effects can significantly interfere with a person's ability . . . to operate motor vehicles." (HHS, 2015) As mentioned in this factor, there is a significant risk with marijuana use and driving. Marijuana was found in 13% of drivers involved in automobile fatal accidents (McCartt, 2015). Furthermore, in a meta-analysis conducted by Li et al. (2011), an association was identified between marijuana use by the driver and an increased risk of getting into a car accident.
The DEA notes that the petitioners only considered whether marijuana creates social problems, and did not consider physiological changes and impacts that also should be evaluated in determining the risk to public health. The HHS and DEA considered the public health impacts of such physiological effects, as discussed in this factor and others above. Marijuana may result in acute cardiovascular toxicity as indicated by recent reviews examining these associations (Hackham, 2015; Panayiotides, 2015). There is a possible association between frequent, long-term marijuana use and increased risk of testicular germ cell cancers and some evidence that chronic marijuana use may lead to lung cancer although the evidence is inconsistent. Furthermore, a more recent risk is the increase in ED visits of children unintentionally exposed to marijuana with increased risk factors for major adverse effects or admission to critical care units in states that have legalized marijuana for medical purposes (Wang et al., 2014).
Factor 7: Its Psychic or Physiological Dependence Liability
Physiological (Physical) Dependence in Humans
The HHS stated that heavy and chronic use of marijuana can lead to physical dependence (DSM-5, 2013; Budney and Hughes, 2006; Haney et al., 1999). Tolerance is developed following repeated administration of marijuana and withdrawal symptoms are observed as following discontinuation of marijuana usage (HHS, 2015).
The HHS mentioned that tolerance can develop to some of marijuana's effects, but does not appear to develop with respect to the psychoactive effects. It is believed that lack of tolerance to psychoactive effects may relate to electrophysiological data demonstrating that chronic Δ9-THC administration does not affect increased neuronal firing in the ventral tegmental area, a brain region that plays a critical role in drug reinforcement and reward (Wu and French, 2000). Humans can develop tolerance to marijuana's cardiovascular, autonomic, and behavioral effects (Jones et al., 1981). Tolerance to some behavioral effects appears to develop with heavy and chronic use, but not with occasional usage. Ramaekers et al. (2009) reported that following acute administration of marijuana, occasional marijuana users still exhibited impairments in tracking and attention tasks whereas performance of heavy users on the these tasks was not affected. In a follow-up study with the same subjects that participated in the study by Ramaekers et al. (2009), a neurophysiological assessment was conducted where event-related potentials (ERPs) were measured using electroencephalography (EEG) (Theunissen et al., 2012). Similar to the earlier results, the heavy marijuana users (n = 11; average of 340 marijuana uses per year) had no changes in their ERPs with the acute marijuana exposure. However, occasional users (n = 10; average of 55 marijuana uses per year) had significant decreases in the amplitude of an ERP component (categorized as P100) on tracking and attention tasks and ERP amplitude change is indicative of a change in brain activity (Theunissen et al., 2012).
The HHS indicated that down-regulation of cannabinoid receptors may be a possible mechanism for tolerance to marijuana's effects (Hirvonen et al., 2012; Gonzalez et al., 2005; Rodriguez de Fonseca et al., 1994; Oviedo et al., 1993).
As indicated by the HHS, the most common withdrawal symptoms in heavy, chronic marijuana users are sleep difficulties, decreased appetite or weight loss, irritability, anger, anxiety or nervousness, and restlessness (Budney and Hughes, 2006; Haney et al., 1999). As reported by HHS, most marijuana withdrawal symptoms begin within 24-48 hours of discontinuation, peak within 4-6 days, and last for 1-3 weeks.
The HHS pointed out that the American Psychiatric Association's (APA's) Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5) included a list of withdrawal symptoms following marijuana [cannabis] use (DSM-5, 2013). The DEA notes that a DSM-5 working group report indicated that marijuana withdrawal symptoms were added to DSM-5 (they were not previously included in DSM-IV) because marijuana withdrawal has now been reliably presented in several studies (Hasin et al., 2013). In short, marijuana withdrawal signs are reported in up to one-third of regular users and between 50% and 90% of heavy users (Hasin et al., 2013). According to DSM-5 criteria, in order to be characterized as having marijuana withdrawal, an individual must develop at least three of the seven symptoms within one week of decreasing or stopping the heavy and prolonged use (DSM-5, 2013). These seven symptoms are: (1) Irritability; anger or aggression, (2) nervousness or anxiety, (3) sleep difficulty, (4) decreased appetite or weight loss, (5) restlessness, (6) decreased mood, (7) somatic symptoms causing significant discomfort (DSM-5, 2013).
Psychological (Psychic) Dependence in Humans
High levels of psychoactive effects such as positive reinforcement correlate with increased marijuana abuse and dependence (Scherrer et al., 2009; Zeiger et al., 2010). Epidemiological marijuana use data reported by NSDUH, MTF, and TEDS support this assertion as presented in the HHS 2015 review of marijuana and updated by the DEA. According to the findings in the 2014 NSDUH survey, an estimated 9.2 million individuals 12 years and older used marijuana daily or almost daily (20 or more days within the past month). In the 2015 MTF report, daily marijuana use (20 or more days within the past 30 days) in 8th, 10th, and 12th graders is 1.1%, 3.0%, and 6.0%, respectively.
The 2014 NSDUH report stated that 4.2 million persons were classified with dependence on or abuse of marijuana in the past year (representing 1.6% of the total population age 12 or older, and 59.0% of those classified with illicit drug dependence or abuse) based on criteria specified in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV). Furthermore, of the admissions to licensed substance abuse facilities, as presented in TEDS, marijuana/hashish was the primary substance of abuse for; 18.3% (352,297) of 2011 admissions; 17.5% (315,200) of 2012 admissions; and 16.8% (281,991) of 2013 admissions. Of the 281,991 admissions in 2013 for marijuana/hashish as the primary substance, 24.3% used marijuana/hashish daily. Among admissions to treatment for marijuana/hashish as the primary substance in 2013, 27.4% were ages 12 to 17 years and 29.7% were ages 20 to 24 years. Start Printed Page 53761
Petitioners' Major Comment in Relation to Factor 7 and the Government's Response
(1) The petitioners stated, "There is no severe physical withdrawal syndrome associated with cannabis. Cannabis addiction is amenable to treatment." (Exhibit B, page 10). The petitioners further indicated that marijuana "may be psychologically addictive, but much less so than other Scheduled [sic] II drugs," (Exhibit B, page 10) and that there is a low risk of dependence associated with marijuana use. Petitioners further stated in Exhibit B, page 23, "Cannabis has low relative dependence risk and does not reach the severity associated with other drugs."
The HHS states that marijuana withdrawal syndrome "appears to be mild compared to classical alcohol and barbiturate withdrawal syndromes" and is similar in magnitude and time course to tobacco withdrawal syndrome.
DSM-5 now recognizes and describes a marijuana [cannabis] withdrawal syndrome. The lifetime risk of dependence to marijuana is approximately 9% among heavy or long-term users (Volkow et al., 2014). Marijuana results in tolerance and withdrawal as described earlier in this Factor 7. The data from NSDUH indicate that there is constant desire for marijuana as noted by the consistently high numbers of current daily users in adults and adolescents. Marijuana use also persists despite problems associated with the drug. Changes in IQ have been noted in adolescent-onset, chronic or dependent marijuana users, in addition to withdrawal symptoms. However, marijuana use has not declined in the time that usage of this drug has been monitored. Additionally, there has been an increase in content of the primary psychoactive chemical, Δ9-THC, in marijuana samples analyzed by the University of Mississippi's Potency Monitoring Project, suggesting preference for marijuana strains with higher levels of Δ9-THC.
Factor 8: Whether the Substance is an Immediate Precursor of a Substance Already Controlled Under the CSA
Marijuana is not an immediate precursor of another controlled substance.
Determination
After consideration of the eight factors discussed above and of the HHS's Recommendation, the DEA finds that marijuana meets the three criteria for placing a substance in schedule I of the CSA under 21 U.S.C. 812(b)(1):
1. Marijuana has a high potential for abuse.
The HHS concluded that marijuana has a high potential for abuse based on a large number of people regularly using marijuana, its widespread use, and the vast amount of marijuana that is available through illicit channels.
Marijuana is the most abused and trafficked illicit substance in the United States. Approximately 22.2 million individuals in the United States (8.4% of the United States population) were past month users of marijuana according to the 2014 NSDUH survey. A 2015 national survey (Monitoring the Future) that tracks drug use trends among high school students showed that by 12th grade, 21.3% of students reported using marijuana in the past month, and 6.0% reported having used it daily in the past month. In 2011, SAMHSA's Drug Abuse Warning Network (DAWN) reported that marijuana was mentioned in 36.4% of illicit drug-related emergency department (ED) visits, corresponding to 455,668 out of approximately 1.25 million visits. The Treatment Episode Data Set (TEDS) showed that 16.8% of non-private substance-abuse treatment facility admissions in 2013 were for marijuana as the primary drug.
Marijuana has dose-dependent reinforcing effects that encourage its abuse. Both clinical and preclinical studies have demonstrated that marijuana and its principle psychoactive constituent, Δ9-THC, possess the pharmacological attributes associated with drugs of abuse. They function as discriminative stimuli and as positive reinforcers to maintain drug use and drug-seeking behavior. Additionally, use of marijuana can result in psychological dependence.
2. Marijuana has no currently accepted medical use in treatment in the United States.
The HHS stated that the FDA has not approved an NDA for marijuana. The HHS noted that there are opportunities for scientists to conduct clinical research with marijuana and there are active INDs for marijuana, but marijuana does not have a currently accepted medical use in the United States, nor does it have an accepted medical use with severe restrictions.
FDA approval of an NDA is not the sole means through which a drug can be determined to have a "currently accepted medical use" under the CSA. Applying the five-part test summarized below, a drug has a currently accepted medical use if all of the following five elements have been satisfied. As detailed in the HHS evaluation and as set forth below, none of these elements has been fulfilled for marijuana:
i. The drug's chemistry must be known and reproducible.
Chemical constituents including Δ9-THC and other cannabinoids in marijuana vary significantly in different marijuana strains. In addition, the concentration of Δ9-THC and other cannabinoids may vary between strains. Therefore the chemical composition among different marijuana samples is not reproducible. Due to the variation of the chemical composition in marijuana strains, it is not possible to derive a standardized dose. The HHS does advise that if a specific Cannabis strain is cultivated and processed under controlled conditions, the plant chemistry may be consistent enough to derive standardized doses.
ii. There must be adequate safety studies.
There are not adequate safety studies on marijuana for use in any specific, recognized medical condition. The considerable variation in the chemistry of marijuana results in differences in safety, biological, pharmacological, and toxicological parameters amongst the various marijuana samples.
iii. There must be adequate and well-controlled studies proving efficacy.
There are no adequate and well-controlled studies that determine marijuana's efficacy. In an independent review performed by the FDA of publicly available clinical studies on marijuana (FDA, 2015), FDA concluded that these studies do not have enough information to "currently prove efficacy of marijuana" for any therapeutic indication.
iv. The drug must be accepted by qualified experts.
At this time, there is no consensus of opinion among experts concerning the medical utility of marijuana for use in treating specific recognized disorders.
v. The scientific evidence must be widely available.
The currently available data and information on marijuana is not sufficient to address the chemistry, pharmacology, toxicology, and effectiveness. The scientific evidence regarding marijuana's chemistry with regard to a specific cannabis strain that could be formulated into standardized and reproducible doses is not currently available.
3. There is a lack of accepted safety for use of marijuana under medical supervision.
Currently, there are no FDA-approved marijuana products. The HHS also concluded that marijuana does not have a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions. According to the HHS, the FDA is unable to conclude that marijuana has an acceptable level of Start Printed Page 53762 safety in relation to its effectiveness in treating a specific and recognized disorder due to lack of evidence with respect to a consistent and reproducible dose that is contamination free. The HHS indicated that marijuana research investigating potential medical use should include information on the chemistry, manufacturing, and specifications of marijuana. The HHS further indicated that a procedure for delivering a consistent dose of marijuana should also be developed. Therefore, the HHS concluded that marijuana does not have an acceptable level of safety for use under medical supervision.
References
1. Abrams DI, Hilton JF, Reiser RJ, Shade SB, Elbeik TA, Aweeka FT, Benowitz NL, Bredt BM, Kosel B, Aberg JA, Deeks SG, Mitchell TF, Mulligan K, Bacchetti P, McCune JM, Schambelan M (2003). Short-term effects of cannabinoids in patients with HIV-1 infection: A randomized, placebo-controlled clinical trial. Ann Intern Med 139(4):258-266.
2. Adams IB, Martin BR (1996). Cannabis: Pharmacology and toxicology in animals and humans. Addiction 91:1585-1614.
3. Agurell S, Dewey WL, and Willetts RE (eds.) (1984). The Cannabinoids: Chemical, Pharmacologic, and Therapeutic Aspects. New York: Academic Press.
4. Agurell S, Halldin M, Lindgren JE, Ohlsson A, Widman M, Gillespie H, Hollister L (1986). Pharmacokinetics and metabolism of delta-1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev 38(1):21-43.
5. American College of Physicians [ACP]. (2008). Supporting Research Into the Therapeutic Role of Marijuana. Philadelphia: American College of Physicians; 2008: Position Paper (Available from American College of Physicians, 190 N. Independence Mall West, Philadelphia, PA 19106).
6. American Medical Association [AMA]. (2009a). Use of Cannabis for Medical Purposes. Report of the Council on Science and Public Health 3.
7. American Medical Association [AMA]. (2009b). AMA Policy: Medical Marijuana. H-95-952 Medical Marijuana.
8. American Medical Association [AMA]. (2013). 2013 Interim Meeting. American Medical Association House of Delegates (I-13). Accessed at www.ama-assn.org/assets/meeting/2013i/i13-refcommk-annotated.pdf.
9. American Society of Addiction Medicine [ASAM]. (2014). New York Times calls for legalization of marijuana, ASAM strongly objects. Accessed at www.asam.org/docs/default-source/pressreleases/asam-press-release-nytimes-editorial-marijuana-2014-7-27.pdf?sfvrsn=2
10. Andreasson S, Allebeck P, Engström A, Rydberg U (1987). Cannabis and schizophrenia: A longitudinal study of Swedish conscripts. Lancet 1:483-1483.
11. Appendino G, Chianese G, Taglialatela-Scafati O (2011). Cannabinoids: Occurrence and medicinal chemistry. Curr Med Chem 18(7):1085-1099.
12. Asch RH, Smith CG, Siler-Khodr TM, Pauerstein CJ (1981). Effects of delta 9-tetrahydrocannabinol during the follicular phase of the rhesus monkey (Macaca mulatta). J Clin Endocrinol Metab 52(1):50-55.
13. Balster RL (1991). Drug abuse potential evaluation in animals. Br J Addict 86(12):1549-1558.
14. Balster RL, Prescott WR (1992). Delta9-Tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Res 16(1):55-62.
15. Balster RL, Bigelow GE (2003). Guidelines and methodological reviews concerning drug abuse liability assessment. Drug Alcohol Depend 70:S13-S40.
16. Barnett G, Licko V, Thompson T (1985). Behavioral pharmacokinetics of marijuana. Psychopharmacology 85(1):51-56.
17. Benowitz NL, Jones RT (1975). Cardiovascular effects of prolonged delta-9-tetrahydrocannabinol ingestion. Clin Pharmacol Ther 18(3):287-297.
18. Benowitz NL, Jones RT (1981). Cardiovascular and metabolic considerations in prolonged cannabinoid administration in man. J Clin Pharmacol 21(8-9 Suppl):214S-223S.
19. Blanco C, Alderson D, Ogburn E, Grant BF, Nunes EV, Hatzenbuehler ML, Hasin DS (2007). Changes in the prevalence of non-medical prescription drug use and drug use disorders in the United States: 1991-1992 and 2001-2002. Drug Alcohol Depend 90(2-3):252-260.
20. Block RI, Farinpour R, Schlechte JA (1991). Effects of chronic marijuana use on testosterone, luteinizing hormone, follicle stimulating hormone, prolactin and cortisol in men and women. Drug Alcohol Depend 28(2):121-128.
21. Block RI, Farinpour R, Braverman K (1992). Acute effects of marijuana on cognition: relationships to chronic effects and smoking techniques. Pharmacol Biochem Behav 43(3):907-917.
22. Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL (2002). Dose-related neurocognitive effects of marijuana use. Neurology 59:1337-1343.
23. Bolla KI, Eldreth DA, Matochik JA, Cadet JL (2005). Neural substrates of faulty decision-making in abstinent marijuana users. NeuroImage 26:480-492.
24. Bonnet U (2013). Chronic cannabis abuse, delta-9-tetrahydrocannabinol and thyroid function. Pharmacopsychiatry 46(1):35-36.
25. Bouaboula M, Rinaldi M, Carayon P, Carillon C, Delpech B, Shire D, Le Fur G, Casellas P (1993). Cannabinoid-receptor expression in human leukocytes. Eur J Biochem 214(1):173-180.
26. Braida D, Iosue S, Pegorini S, Sala M (2004). Delta9-tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur J Pharmacol 506(1):63-69.
27. Brievogel CS, Childers SR (2000). Cannabinoid agonist signal transduction in rat brain: comparison of cannabinoid agonists in receptor binding, G-protein activation, and adenylyl cyclase inhibition. J Pharmacol Exp Ther 295(1):328-336.
28. Brown TT, Dobs AS (2002). Endocrine effects of marijuana. J Clin Pharmacol 42(11 Suppl):90S-96S.
29. Browne RG, Weisman A (1981). Discriminative stimulus properties of delta 9-tetrahydrocannabinol: mechanistic studies. J Clin Pharmacol 21(8-9 Suppl):227S-234S.
30. Budney AJ, Hughes JR (2006). The cannabis withdrawal syndrome. Curr Opin Psychiatry 19(3):233-238.
31. Capriotti RM, Foltin RW, Brady JV, Fischman MW (1988). Effects of marijuana on the task-elicited physiological response. Drug Alcohol Depend 21(3):183-187.
32. Cascini F, Aiello C, Di Tanna G (2012). Increasing delta-9-tetrahydrocannabinol Δ-9-THC) content in herbal cannabis over time: systematic review and meta-analysis. Curr Drug Abuse Rev 5(1):32-40.
33. Chait LD, Burke KA (1994). Preference for "high" versus low-potency marijuana. Pharmacol Biochem Behav 7:357-364.
34. Cheer JF, Kendall DA, Marsden CA (2000). Cannabinoid receptors and reward in the rat: a conditioned place preference study. Psychopharmacology (Berl) 151(1):25-30.
35. Compton RP, Berning A (2015). Drug and Alcohol Crash Risk. Traffic Safety Facts Research Note. DOT HS 812 117. Washington, DC: National Highway Traffic Safety Administration.
36. Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS (2004). Prevalence of marijuana use disorders in the United States: 1991-1992 and 2001-2002. JAMA 291(17):2114-2121.
37. Cone EJ, Johnson RE, Moore JD, Roache JD (1986). Acute effects of smoking marijuana on hormones, subjective effects and performance in male human subjects. Pharmacol Biochem Behav 24(6):1749-1754.
38. Croxford JL, Yamamura T (2005). Cannabinoids and the immune system: potential for the treatment of inflammatory diseases? J Neuroimmunol 166(1-2):3-18.
39. Daling JR, Doody DR, Sun X, Trabert BL, Weiss NS, Chen C, Biggs ML, Starr JR, Dey SK, Schwartz SM (2009). Association of marijuana use and the incidence of testicular germ cell tumors. Cancer. 115(6):1215-1223.
40. Dalton WS, Martz R, Lemberger L, Rodda BE, Forney RB (1976). Influence of cannabidiol on delta-9-tetrahydrocannabinol effects. Clin Pharmacol Ther 19(3):300-309.
41. Dax EM, Pilotte NS, Adler WH, Nagel JE, Lange WR (1989). The effects of 9-enetetrahydrocannabinol on hormone release and immune function. J Steroid Biochem 34(1-6):263-270. Start Printed Page 53763
42. Day NL, Wagener DK, Taylor PM (1985). Measurement of substance use during pregnancy: methodologic issues. NIDA Res Monogr 59:36-47.
43. Day NL, Leech SL, Goldschmidt L (2011). The effects of prenatal marijuana exposure on delinquent behaviors are mediated by measures of neurocognitive functioning. Neurotoxicol Teratol 33(1):129-136.
44. Day NL, Goldschmidt L, Day R, Larkby C, Richardson GA (2015). Prenatal marijuana exposure, age of marijuana initiation, and the development of psychotic symptoms in young adults. Psychol Med 45(8):1779-1787.
45. Degenhardt L, Hall W, Lynskey M (2003). Testing hypotheses about the relationship between cannabis use and psychosis. Drug Alcohol Depend 71(1):37-48.
46. Degenhardt L, Hall WD, Lynskey M, McGrath J, McLaren J, Calabria B, Whiteford H, Vos T (2009). Should burden of disease estimates include cannabis use as a risk factor for psychosis? PLoS Medicine. 6(9):e1000133.
47. Degenhardt L, Dierker L, Chiu WT, Medina-Mora ME, Neumark Y, Sampson N, Alonso J, Angermeyer M, Anthony JC, Bruffaerts R, et al (2010). Evaluating the drug use "gateway" theory using cross-national data: consistency and associations of the order of initiation of drug use among participants in the WHO World Mental Health Surveys. Drug Alcohol Depend 108(1-2):84-97.
48. De Petrocellis PL, Di Marzo V (2009). An introduction to the endocannabinoid system: from the early to the latest concepts. Best Pract Res Clin Endocrinol Metab 23(1):1-15.
49. Department of Health and Human Services [HHS] (2015). Basis for the recommendation for maintaining marijuana in Schedule I of the Controlled Substances Act.
50. Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R (1992). Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258(5090):1946-1949.
51. Dewey WL, Martin BR, May EL (1984). Cannabinoid stereoisomers: pharmacological effects. In Smith DF. (Ed.) CRC Handbook of stereoisomers: drugs in psychopharmacology, 317-26 (Boca Raton, FL, CRC Press).
52. Drug Enforcement Administration (2015). Drugs of Abuse.
53. DSM-5 (2013). Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. American Psychiatric Association. Washington, DC: American Psychiatric Publishing.
54. Eisenstein TK, Meissler JJ (2015). Effects of cannabinoids on T-cell function and resistance to infection. J Neuroimmune Pharmacol 10(2):204-216.
55. Eldridge JC, Murphy LL, Landfield PW (1991). Cannabinoids and the hippocampal glucocorticoid receptor: recent findings and possible significance. Steroids 56(5):226-231.
56. ElSohly MA, Slade D (2005). Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci 78:539-548.
57. Fant RV, Heishman SJ, Bunker EB, Pickworth WB (1998). Acute and residual effects of marijuana in humans. Pharmacol Biochem Behav 60(4):777-784.
58. Federal Register (1992). "Marijuana Scheduling Petition; Denial of Petition; Remand"—Drug Enforcement Administration, Final Order. Fed Registr 57(59):10499-10508.
59. Federal Register (1999). "Rescheduling of the Food and Drug Administration Approved Product Containing Synthetic Dronabinol [(-)-delta 9-(trans)-Tetrahydrocannabinol] in Sesame Oil and Encapsulated in Soft Gelatin Capsules From Schedule II to Schedule III; Final Rule," Fed Registr 64(127):35928-35930.
60. Federal Register (2001). "Notice of Denial of Petition: Basis for the Recommendation for Maintaining Marijuana in Schedule I of the Controlled Substances Act," Fed Registr 66(75):20038-20076.
61. Fergusson DM, Horwood LJ, Ridder EM (2005). Tests of causal linkages between cannabis use and psychotic symptoms. Addiction. 100(3):354-366.
62. Fontes MA, Bolla KI, Cunha PJ, Almeida PP, Jungerman F, Laranjeira RR, Bressan RA, Lacerda AL (2011). Cannabis use before age 15 and subsequent executive functioning. Br J Psychiatry 198(6):442-447.
63. Fried PA, Watkinson B, Grant A, Knights RM (1980). Changing patterns of soft drug use prior to and during pregnancy: a prospective study. Drug Alcohol Depend 6(5):323-343.
64. Fried PA, Watkinson B (1990). 36- and 48-month neurobehavioral follow-up of children prenatally exposed to marijuana, cigarettes and alcohol. J Dev Behav Pediatr 11:49-58.
65. Fried PA, Watkinson B, Gray R (1992). A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marihuana, cigarettes and alcohol. Neurotoxicol Teratol 14:299-311.
66. Fried PA, Watkinson B, Gray R (1998). Differential effects on cognitive functioning in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol 20(3):293-306.
67. Fried PA (2002). Adolescents prenatally exposed to marijuana: examination of facets of complex behaviors and comparisons with the influence of in utero cigarettes. J Clin Pharmacol 42(11 Suppl):97S-102S.
68. Fried PA, Watkinson B, Gray R (2005). Neurocognitive consequences of marihuana—a comparison with pre-drug performance. Neurotoxicol Teratol 27(2):231-239.
69. Fung M, Gallagher C, Machtay M (1999). Lung and aero-digestive cancers in young marijuana smokers. Tumori 85(2):140-142.
70. Gates P, Jaffe A, Copeland J (2014). Cannabis smoking and respiratory health: consideration of the literature. Respirology 19(5):655-662.
71. Ghozland S, Matthes HW, Simonin F, Filliol D, Kieffer BL, Maldonado R (2002). Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. J Neurosci 22(3):1146-1154.
72. Gold LH, Balster RL, Barrett RL, Britt DT, Martin BR (1992). A comparison of the discriminative stimulus properties of delta 9-tetrahydrocannabinol and CP 55,940 in rats and rhesus monkeys. J Pharmacol Exp Ther 262(2):479-486.
73. Goldschmidt L, Richardson GA, Willford JA, Day NL (2008). Prenatal marijuana exposure and intelligence test performance at age 6. J Am Acad Child Adolesc Psychiatry 47(3):254-263.
74. Goldschmidt L, Richardson GA, Willford JA, Severtson SG, Day NL (2012). School achievement in 14-year-old youths prenatally exposed to marijuana. Neurotoxicol Teratol 34(1):161-167.
75. Gong H Jr, Tashkin DP, Simmons MS, Calvarese B, Shapiro BJ (1984). Acute and subacute bronchial effects of oral cannabinoids. Clin Pharmacol Ther 35(1):26-32.
76. Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR (2006). Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res 1071(1):10-23.
77. Gonsiorek W, Lunn C, Fan X, Narula S, Lundell D, Hipkin RW. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Mol Pharmacol 57(5):1045-1050.
78. Gonzalez R (2007). Acute and non-acute effects of cannabis on brain functioning and neuropsychological performance. Neuropsychol Rev 17(3):347-361.
79. Gonzalez S, Cebeira M, Fernandez-Ruiz J (2005). Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav 81(2):300-318.
80. Gore RL, Earleywine M (2007). Marijuana's perceived addictiveness: A survey of clinicians and researchers. In M. Earleywine, (Ed.) Pot politics: The cost of prohibition. New York: Oxford University Press.
81. Griffith-Lendering MF, Wigman JT, Prince van Leeuwen A, Huijbregts SC, Huizink AC, Ormel J, Verhulst FC, van Os J, Swaab H, Vollebergh WA (2013). Cannabis use and vulnerability for psychosis in early adolescence—a TRAILS study. Addiction 108(4):733-740.
82. Grotenhermen F (2003). Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 42(4):327-360.
83. Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE (2012). Age of onset of marijuana use and executive function. Psychol Addict Behav 26(3):496-506.
84. Hackam DG (2015). Cannabis and stroke: systematic appraisal of case reports. Stroke 46(3):852-856. Start Printed Page 53764
85. Hall WD, Lynskey M (2005). Is cannabis a gateway drug? Testing hypotheses about the relationship between cannabis use and the use of other illicit drugs. Drug Alcohol Rev 24(1):39-48.
86. Hall W, Degenhardt L (2014). The adverse health effects of chronic cannabis use. Drug Test Anal 6(1-2):39-45.
87. Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW (1999). Abstinence symptoms following smoked marijuana in humans. Psychopharmacology (Berl) 141(4):395-404.
88. Harder S, Rietbrock S (1997). Concentration-effect relationship of delta-9-tetrahydrocannabinol and prediction of psychotropic effects after smoking marijuana. International Journal of Clinical Pharmacology and Therapeutics. 35(4):155-159.
89. Hasin DS, O'Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, Compton WM, Crowley T, Ling W, Petry NM, Schuckit M, Grant BF (2013). DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry 170(8):834-851.
90. Heishman SJ, Huestis MA, Henningfield JE, Cone EJ (1990). Acute and residual effects of marijuana: profiles of plasma THC levels, physiological, subjective, and performance measures. Pharmacol Biochem Behav 37(3):561-565.
91. Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC (1990). Cannabinoid receptor localization in brain. Proc Natl Acad Sci 87:1932-1936.
92. Herkenham M (1992). Cannabinoid receptor localization in brain: relationship to motor and reward systems. Ann N Y Acad Sci 654:19-32.
93. Herning RI, Hooker WD, Jones RT (1986). Tetrahydrocannabinol content and differences in marijuana smoking behavior. Psychopharmacology (Berl) 90(2):160-162.
94. Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB (2012). Reversible and regionally selective downregulation of brain cannabinoid CB 1 receptors in chronic daily cannabis smokers. Mol Psychiatry 17(6):643-649.
95. Hively RL, Mosher WA, Hoffman FW (1966). Isolation of trans-Δ9-tetrahydrocannabinol from marihuana. J Am Chem Soc 88:1832-1833.
96. Hollister LE, Gillespie HK (1973). Delta-8- and delta-9-tetrahydrocannabinol comparison in man by oral and intravenous administration. Clin Pharmacol Ther 14(3):353-357.
97. Hollister LE (1986). Health aspects of cannabis. Pharmacological Rev 3:1-20.
98. Hollister LE (1988). Cannabis (Literature review). Acta Psychiatr Scand (Suppl) 78:108-118.
99. Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ (2004). Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology 47 Suppl:345-358.
100. Huang YH, Zhang ZF, Tashkin DP, Feng B, Straif K, Hashibe K (2015). An epidemiologic review of marijuana and cancer: an update. Cancer Epidemiol Biomarkers Prev 24(1):15-31.
101. Huestis MA, Sampson AH, Holicky BJ, Benningfield JE, Cone EJ (1992a). Characterization of the absorption phase of marijuana smoking. Clin Pharmacol Ther 52:31-41.
102. Huestis MA, Benningfield JE, Cone EJ (1992b). Blood Cannabinoids. 1. Absorption of THC and formation of 11-0H-THC and THC COOH during and after smoking marijuana. J Anal Toxicol 16(5):276-282.
103. Hunt CA, Jones RT (1980). Tolerance and disposition of tetrahydrocannabinol in man. J Pharmacol Exp Ther 215(1):35-44.
104. Ilan AB, Gevins A, Coleman M, ElSohly MA, de Wit H (2005). Neurophysiological and subjective profile of marijuana with varying concentrations of cannabinoids. Behav Pharmacol 16(5-6):487-496.
105. Institute of Medicine (1982). Division of Health Sciences Policy. Marijuana and Health: Report of a Study by a Committee of the Institute of Medicine, Division of Health Sciences Policy. Washington, DC: National Academy Press, 1982.
106. Institute of Medicine (1999). Division of Neuroscience and Behavioral Health. Marijuana and Medicine: Assessing the Science Base. Washington, DC: National Academy Press, 1999.
107. Johansson E, Halldin MM, Agurell S, Hollister LE, Gillespie HK (1989). Terminal elimination plasma half-life of delta 1-tetrahydrocannabinol (delta 1-THC) in heavy users of marijuana. Eur J Clin Pharmacol 37(3):273-277.
108. Jones RT, Benowitz NL, Heming RI (1981). Clinical relevance of cannabis tolerance and dependence. J Clin Pharmacol 21:143S-152S.
109. Jones RT (2002). Cardiovascular system effects of marijuana. J Clin Pharmacol 42(11Suppl):58S-63S.
110. Justinova Z, Tanda G, Redhi GH, Goldberg SR (2003). Self-administration of delta9-tetrahydrocannabinol (THC) by drug naïve squirrel monkeys. Psychopharmacology (Berl) 169(2):135-140.
111. Justinova Z, Tanda G, Munzar P, Goldberg SR (2004). The opioid antagonist naltrexone reduces the reinforcing effects of Delta 9 tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology (Berl) 173(1-2):186-194.
112. Kandel D (1975). Stages in adolescent involvement in drug use. Science 190:912-914.
113. Kandel DB, Chen K (2000). Types of marijuana users by longitudinal course. J Stud Alcohol 61(3):367-378.
114. Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA (1974). Cannabidiol interferes with the effects of delta 9-tetrahydrocannabinol in man. Eur J Pharmacol 28(1):172-177.
115. Karniol IG, Shirakawa I, Takahashi RN, Knobel E, Musty RE (1975). Effects of delta9-tetrahydrocannabinol and cannabinol in man. Pharmacology 13(6):502-512.
116. Keen L 2nd, Pereira D, Latimer W (2014). Self-reported lifetime marijuana use and interleukin-6 levels in middle-aged African Americans. Drug Alcohol Depend 140:156-160.
117. Kirk JM, de Wit H (1999). Responses to oral delta9-tetrahydrocannabinol in frequent and infrequent marijuana users. Pharmacol Biochem Behav 63(1):137-142.
118. Kuepper R, van Os J, Lieb R, Wittchen HU, Henquet C (2011). Do cannabis and urbanicity co-participate in causing psychosis? Evidence from a 10-year follow-up cohort study. Psychol Med 41(10):2121-2129.
119. Kurzthaler I, Hummer M, Miller C, Sperner-Unterweger B, Gunther V, Wechdorn H, Battista HJ, Fleischhacker WW (1999). Effects of cannabis use on cognitive functions and driving ability. J Clin Psychiatry 60(6):395-399.
120. Lacson JC, Carroll JD, Tuazon E, Castelao EJ, Bernstein L, Cortessis VK (2012). Population-based case-control study of recreational drug use and testis cancer risk confirms an association between marijuana use and nonseminoma risk. Cancer 118:5374-5383.
121. Lee MH, Hancox RJ (2011). Effects of smoking cannabis on lung function. Exp Rev Resp Med 5(4):537-546.
122. Lemberger L, Silberstein SD, Axelrod J, Kopin IJ (1970). Marihuana: studies on the disposition and metabolism of delta-9-tetrahydrocannabinol in man. Science 70:1320-1322.
123. Lemberger L, Weiss JL, Watanabe AM, Galanter IM, Wyatt RJ, Cardon PV (1972a). Delta-9-tetrahydrocannabinol: temporal correlation of the psychological effects and blood levels after various routes of administration. New Eng J Med 286(13):685-688.
124. Lemberger L, Crabtree RE, Rowe HM (1972b). 11-Hydroxy-Δ9-tetrahydrocannabinol: pharmacology, disposition and metabolism of a major metabolite of marihuana in man. Science 77:62-63.
125. Lemberger L, Rubin A (1975). The physiologic disposition of marihuana in man. Life Sci 17:1637-1642.
126. Li M-C, Brady JE, DiMaggio CJ, Lusardi AR, Tzong, KY, Li G (2012). Marijuana use and motor vehicle crashes. Epidemiologic Rev 34:65-72.
127. Liguori A, Gatto CP, Robinson JH (1998). Effects of marijuana on equilibrium, psychomotor performance, and simulated driving. Behav Pharmacol 9(7):599-609.
128. Lisdahl KM, Price JS (2012). Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. J Int Neuropsychol Soc 18(4):678-688.
129. Lyons MJ, Bar JL, Panizzon MS, Toomey R, Eisen S, Xian H, Tsuang MT (2004). Neuropsychological consequences of regular marijuana use: a twin study. Psychol Med 34(7):1239-1250.
130. Mackie K, Lai Y, Westenbroek R, Mitchell R (1995). Cannabinoids activate an inwardly rectifying potassium Start Printed Page 53765 conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci 15(10):6552-6561.
131. Maldonado R (2002). Study of cannabinoid dependence in animals. Pharmacol Ther 95(2):153-164.
132. Malinowska B, Baranowska-Kuczko M, Schlicker E (2012). Triphasic blood pressure responses to cannabinoids: do we understand the mechanism? Br J Pharmacol 165(7):2073-2088.
133. Manrique-Garcia E, Zammit S, Dalman C, Hemmingson T, Andreasson S, Allebeck P (2012). Cannabis, schizophrenia and other non-affective psychoses: 35 years of follow-up of a population-based cohort. Psychol Med 42(6):1321-1328.
134. Maremmani I, Lazzeri A, Pacini M, Lovrecic M, Placidi GF, Perugi G (2004). Diagnostic and symptomatological features in chronic psychotic patients according to cannabis use status. J Psychoactive Drugs 36(2):235-241.
135. McCartt AT (2015). Marijuana and driving in the United States: prevalence, risks, and laws. Presented at the Casualty Actuarial Society Spring Meeting. Colorado Springs, CO. May 19, 2015.
136. McMahon LR, Ginsburg BC, Lamb RJ (2008). Cannabinoid agonists differentially substitute for the discriminative stimulus effects of Delta(9)-terahydrocannabinol in C57BL/6J mice. Psychopharmacology (Berl) 198(4):487-495.
137. McMahon LR (2009). Apparent affinity estimates of rimonabant in combination with anandamide and chemical analogs of anandamide in rhesus monkeys discriminating Delta9-tetrahydrocannabinol. Psychopharmacology (Berl) 203(2):219-228.
138. Mechoulam R (1973). Cannabinoid chemistry. In Mechoulam, R. (ed.) Marijuana, pp.2-88 (New York, NY, Academic Press, Inc.).
139. Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE., Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z (1995). Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50(1):83-90.
140. Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, Ross SA, Khan IA, ElSohly MA (2010). Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci 55(5):1209-1217.
141. Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, McDonald K, Ward A, Poulton R, Moffitt TE (2012). Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA 109(40):E2657-E2664.
142. Mendelson JH, Mello NK (1984). Effects of marijuana on neuroendocrine hormones in human males and females. NIDA Res Monogr 44:97-114.
143. Messinis L, Kyprianidou A, Malefaki S, Papathanasopoulos P (2006). Neuropsychological deficits in long-term frequent cannabis users. Neurology 66:737-739.
144. Minozzi S, Davoli M, Bargagli AM, Amato L, Vecchi S, Perucci CA (2010). An overview of systematic reviews on cannabis and psychosis: discussing apparently conflicting results. Drug Alcohol Rev 29(3):304-317.
145. Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE (2001). Triggering myocardial infarction by marijuana. Circulation 103:2805-2809.
146. Nace EP, Meyers AL, Rothberg JM, Maleson F (1975). Addicted and nonaddicted drug users. A comparison of drug usage patterns. Arch Gen Psychiatry 32(1):77-80.
147. Oviedo A, Glowa J, Herkenham M (1993). Chronic cannabinoid administration alters cannabinoid receptor binding in rat brain: a quantitative autoradiographic study. Brain Res 616:293-302.
148. Pacher P, Batkai S, Kunos G (2006). The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58(3):389-462.
149. Panayiotides IM (2015). What is the association between cannabis consumption and cardiovascular complications. Subst Abuse 9:1-3.
150. Pelayo-Teran JM, Suarez-Pinilla P, Chadi N, Crespo-Pacorro B (2012). Gene-environment interactions underlying the effect of cannabis in first episode psychosis. Curr Pharm Des 18(32):5024-5035.
151. Piomelli D (2005). The endocannabinoid system: a drug discovery perspective. Curr Opin Investig 6(7):672-679.
152. Pletcher MJ, Vittinghoff E, Kalhan R, Richman J, Safford M, Sidney S, Lin F, Kertesz S (2012). Association between marijuana exposure and pulmonary function over 20 years. Journal of the American Medical Association. 307(2):173-181.
153. Pollastro F, Taglialatela-Scafati O, Allara M, Munoz E, Di Marzo V, De Petrocellis L, Appendino G (2011). Bioactive prenylogous cannabinoid from fiber hemp (Cannabis sativa). J Nat Prod. 74(9):2019-2022
154. Pope HG Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D (2002). Cognitive measures in long-term cannabis users. J Clin Pharmacol 42(11 Suppl):41S-47S.
155. Radwan MM, ElSohly MA, Slade D, Ahmed SA, Khan IA, Ross SA (2009). Biologically active cannabinoids from high-potency Cannabis sativa. J Nat Prod 72(5):906-911.
156. Ramaekers JG, Berghaus G, van Laar M, Drummer OH (2004). Dose related risk of motor vehicle crashes after cannabis use. Drug and Alcohol Dependence. 73(2):109-119.
157. Ramaekers JG, Kauert G, van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR (2006). High-potency marijuana impairs executive functions and inhibitory motor control. Neuropsychopharmacology 31(10):2296-2303.
158. Ramaekers JG, Kauert G, Theunissen EL, Toennes SW., Moeller MR (2009). Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol 23(3):266-277.
159. Riggs PK, Vaida F, Rossi SS, Sorkin LS, Gouaux B, Grant I, Ellis RJ (2012). A pilot study of the effects of cannabis on appetite hormones in HIV-infected adult men. Brain Res 1431:46-52.
160. Rodriguez de Fonseca F, Gorriti MA, Fernandez-Ruiz JJ, Palomo T, Ramos JA (1994). Downregulation of rat brain cannabinoid binding sites after chronic delta 9-tetrahydrocannabinol treatment. Pharmacol Biochem Behav 47(1):33-40.
161. Roth MD, Arora A, Barsky SH, Kleerup EC, Simmons M, Tashkin DP (1998). Airway inflammation in young marijuana and tobacco smokers. American Journal of Respiratory and Crit Care Med 157:928-937.
162. Roth MD, Tashkin DP, Whittaker KM, Choi R, Baldwin GC (2005). Tetrahydrocannabinol suppresses immune function and enhances HIV replication in the huPBL-SCID mouse. Life Sci 77(14):1711-1722.
163. Russo E, Mathre ML, Byrne A, Velin R, Bach PJ, Sanchez-Ramos J, Kirlin KA (2001). Chronic cannabis use in the compassionate investigational new drug program: An examination of benefits and adverse effects of legal clinical cannabis. J Cannabis Ther 2:3-57.
164. Sarfaraz S, Afaq F, Adhami VM, Mukhtar H (2005). Cannabinoid receptor as a novel target for the treatment of prostate cancer. Cancer Res 65(5):1635-1641.
165. Scherrer JF, Grant JD, Duncan AE, Sartor CE, Haber JR, Jacob T, Bucholz KK (2009). Subjective effects to cannabis are associated with use, abuse and dependence after adjusting for genetic and environmental influences. Drug Alcohol Depend 105(1-2):76-82.
166. Schiffman J, Nakamura B, Earleywine MJ, LaBrie J (2005). Symptoms of schizotypy precede cannabis use. Psychiatry Res 134(1):37-42.
167. Schimmelmann BG, Conus P, Cotton SM, Kupferschmid S, Karow A, Schultze-Lutter F, McGorry PD, Lambert M (2011). Cannabis use disorder and age at onset of psychosis—a study in first episode patients. Schizophr Res 129(1):52-56.
168. Schreiner AM, Dunn ME (2012). Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: a meta-analysis. Exp Clin Psychopharmacol 20(5):420-429.
169. Sexton M, Cudaback E, Abdullah RA, Finnell J, Mischley LJ, Rozga M, Lichtman AH, Stella N (2014). Cannabis use by individuals with multiple sclerosis: effects on specific immune parameters. Inflammopharmacology 22(5):295-303.
170. Sidney S (2002). Cardiovascular consequences of marijuana use. J Clin Pharmacol 42(11Suppl):64S-70S. Start Printed Page 53766
171. Solinas M, Panlilio LV, Justinova Z, Yasar S, Goldberg SR (2006). Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats. Nat Protoc 1(3):1194-1206.
172. Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B,Vendetti J (2002). Marijuana Treatment Project Research Group. Cognitive functioning of long-term heavy cannabis users seeking treatment. Journal of the American Medical Association 287(9):1123-1131.
173. Substance Abuse and Mental Health Services Administration (2013). Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits. HHS Publication No. (SMA) 13-4760, DAWN Series D-39. Rockville, MD.
174. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality (2015a). Results from the 2014 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD.
175. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality (2015b). Treatment Episode Data Set (TEDS): 2003-2013. National Admissions to Substance Abuse Treatment Services. BHSIS Series S-75, HHS Publication No. (SMA) 15-4934. Rockville, MD.
176. Tait RJ, MacKinnon A, Christensen H (2011). Cannabis use and cognitive functioning: 8-year trajectory in a young adult cohort. Addiction 106(12):2195-2203.
177. Tanasescu R, Constantinescu CS (2010). Cannabinoids and the immune system: an overview. Immunobiology 215(8):588-597.
178. Tanda G, Munzar P, Goldberg SR (2000). Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci 3(11):1073-1074.
179. Tanda G, Goldberg SR (2003). Cannabinoids: reward, dependence, and underlying neurochemical mechanisms—a review of recent preclinical data. Psychopharmacology (Berl) 169(2):115-134.
180. Tashkin DP (2005). Smoked marijuana as a cause of lung injury. Monaldi Arch Chest Dis 63(2):93-l00.
181. Tashkin DP, Zhang ZF, Greenland S, Cozen W, Mack TM, and Morgenstern H (2006). Marijuana use and lung cancer: results of a case-control study. American Thoracic Society International Conference. Abstract A777.
182. Theunissen EL, Kauert GF, Toennes SW., Moeller MR, Sambeth A, Blanchard MM, Ramaekers JG (2012). Neurophysiological functioning of occasional and heavy cannabis users during THC intoxication. Psychopharmacology (Berl) 220(2):341-350.
183. Trabert B, Sigurdson AJ, Sweeney AM, Strom SS, McGlynn KA (2011). Marijuana use and testicular germ cell tumors. Cancer 117(4):848-853.
184. Twitchell W, Brown S, Mackie K (1997). Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol 78(1):43-50.
185. U.S. Food and Drug Administration (FDA), Center for Drug and Evaluation Research, Controlled Substances Staff (2015). The Medical Application of Marijuana: A Review of Published Clinical Studies. March 19, 2015.
186. van der Meer FJ, Velthorst E, Meijer CJ, Machielsen MW, de Haan L (2012). Cannabis use in patients at clinical high risk of psychosis: impact on prodromal symptoms and transition to psychosis. Curr Pharm Des 18(32):5036-5044.
187. van Gastel WA, Wigman JT, Monshouwer K, Kahn RS, van Os J, Boks MP, Volleburgh WA (2012). Cannabis use and subclinical positive psychotic experiences in early adolescence: findings from a Dutch survey. Addiction 107(2):381-387.
188. Van Gundy K, Rebellon CJ (2010). A Life-course Perspective on the "Gateway Hypothesis." J Health Soc Behav 51(3):244-259.
189. van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H. (2002). Cannabis use and psychosis: a longitudinal population-based study. Am J Epi 156(4):319-327.
190. Vann RE, Gamage TF, Warner JA, Marshall EM, Taylor NL, Martin BR, Wiley JL (2008). Divergent effects of cannabidiol on the discriminative stimulus and place conditioning effects of Delta(9)-tetrahydrocannabinol. Drug Alcohol Depend 94(1-3):191-198.
191. Volkow ND, Baler RD, Compton WM, Weiss SR (2014). Adverse health effects of marijuana use. N Engl J Med 370(23):2219-2227.
192. von Sydow K, Lieb R, Pfister H, Hofler M, Wittchen HU (2002). What predicts incident use of cannabis and progression to abuse and dependence? A 4-year prospective examination of risk factors in a community sample of adolescents and young adults. Drug Alcohol Depend 68(1):49-64.
193. Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H (2002). Comparison of the subjective effects of Delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl) 161(4):331-339.
194. Wagner JA, Varga K, Kunos G (1998). Cardiovascular actions of cannabinoids and their generation during shock. J Mol Med 76(12):824-836.
195. Wang GS, Roosevelt G, Heard K (2013). Pediatric marijuana exposures in a medical marijuana state. JAMA Pediatr 167(7):630-633.
196. Wang GS, Roosevelt G, Le Lait MC, Martinez EM, Bucher-Bartelson B, Bronstein AC, Heard K (2014). Association of unintended pediatric exposures with decriminalization of marijuana in the United States. Ann Emerg Med 63(6):684-689.
197. Wang T, Collet JP, Shapiro S, Ware MA (2008). Adverse effects of medical cannabinoids: a systematic review. CMAJ 178(13):1669-1678.
198. Wesson DR, Washburn P (1990). Current patterns of drug abuse that involve smoking. NIDA Res Monogr 99:5-11.
199. Whitehill JM, Rivara FP, Moreno MA (2014). Marijuana-using drivers, alcohol-using drivers, and their passengers: prevalence and risk factors among underage college students. JAMA Pediatr 168(7):618-624.
200. Wiley JL, Barrett RL, Britt DL, Balster RL, Martin BR (1993). Discriminative stimulus effects of delta 9-tetrahydrocannabinol and delta 9-11-tetrahydrocannabinol in rats and rhesus monkeys. Neuropharmacology 32(4):359-365.
201. Wiley JL, Huffman JW, Balster RL, Martin BR (1995). Pharmacological specificity of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rhesus monkeys. Drug Alcohol Depend 40(1):81-86.
202. Wilkinson ST, Radhakrishnan R, D'Souza DC (2014). Impact of cannabis use on the development of psychotic disorders. Curr Addict Rep 1(2):115-128.
203. Wilson FA, Stimpson JP, Pagán JA (2014). Fatal crashes from drivers testing positive for drugs in the U.S., 1993-2010. Public Health Rep 129(4):342-350.
204. Wu X, French ED (2000). Effects of chronic delta9-tetrahydrocannabinol on rat midbrain dopamine neurons: an electrophysiological assessment. Neuropharmacology 39(3):391-398.
205. Zeiger JS, Haberstick BC, Corley RP, Ehringer MA, Crowley TJ, Hewitt JK, Hopfer CJ, Stallings MC, Young SE., Rhee SH (2010). Subjective effects to marijuana associated with marijuana use in community and clinical subjects. Drug Alcohol Depend 109(1-3):161-66.
206. Zhang ZF, Morgenstern H, Spitz MR, Tashkin DP, Yu GP, Marshall JR, Hsu TC, Schantz, SP (1999). Marijuana use and increased risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol Biomarkers Prev 8(12):1071-1078.
207. Zhang LR, Morgenstern H, Greenland S, Chang SC, Lazarus P, Teare MD, Woll PJ, Orlow I, Cox B on behalf of the Cannabis and Respiratory Disease Research Group of New Zealand, Brhane Y, Liu G, Hung RJ (2015). Cannabis smoking and lung cancer risk: Pooled analysis in the International Lung Cancer Consortium. Int J Cancer 136(4):894-903.
208. Zwardi AW, Shirakawa I, Finkelfarb E, Karniol IG (1982). Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subject. Psychopharmcology (Berl) 76(3):245-250.
[FR Doc. 2016-17954 Filed 8-11-16; 8:45 am]
BILLING CODE 4410-09-P
Corey Is Trying to Cut Back on His Consumption of Caffeinated Beverages. He Will Not Experience:
Source: https://www.federalregister.gov/documents/2016/08/12/2016-17954/denial-of-petition-to-initiate-proceedings-to-reschedule-marijuana
0 Response to "Corey Is Trying to Cut Back on His Consumption of Caffeinated Beverages. He Will Not Experience:"
Post a Comment